Abstract
Background
Few data are available on the clinical features and outcomes of Chinese patients with systemic immunoglobulin light-chain (AL) amyloidosis. The aim of this study is to reveal the clinical picture and risk factors of disease progression in a large cohort of Chinese patients with AL amyloidosis.
Methods
Patients in the Jinling Hospital amyloidosis registry from 2003 to 2011 were studied. The clinical and laboratory information were collected from first presentation to death or until the last available clinical follow-up. The patients' survival and renal outcomes were analyzed, and the relationships between the clinical parameters and survival were also assessed.
Results
A total of 231 patients were enrolled in this study, all the patients studied had renal involvement. One hundred and fifty-three (66.2%) were male, and the median age at diagnosis was 56 years. A total of 198 (85.7%) cases had light-chain λ-type. One hundred and forty-seven (63.6%) cases presented as nephrotic syndrome (NS), and 25% of patients had renal insufficiency at diagnosis. Liver involvement and NS appeared to be more common in patients of κ-type amyloidosis, and renal impairment is more severe in κ-type amyloidosis. The median survival time of all patients was 36.3 months, and the 1-, 2-, 3- and 5-year cumulative survival rates were 67, 53, 48 and 35%, respectively. Multivariate COX analysis showed that age, hepatic involvement and heart involvement can significantly influence survival in these patients. The median time that patients remained dialysis free was 50 months. The percentage of patients that remained dialysis free at 1, 2, 3 and 5 years were 78, 69, 62 and 37%, respectively. Multivariate COX analysis showed that serum creatinine and hypotension were the important risk factors of renal failure.
Conclusion
λ-Type is the most dominant type of AL amyloidosis in Chinese patients. The survival of patients with AL amyloidosis is poor. The risk factors included heart and hepatic involvement, hypotension and impairment of renal function. The high serum creatinine level and hypotension at diagnosis are associated with poor renal outcome.
Keywords: AL amyloidosis, clinical characteristic, prognosis, risk factors
Introduction
Immunoglobulin light-chain (AL) amyloidosis, caused by misfolded AL proteins that aggregate and deposit as unique fibrils, is usually associated with an underlying monoclonal plasma dyscrasia [1–3]. The deposition of insoluble amyloid fibrils in vital organs, such as the heart, kidney, liver and nerves, can lead to progressive organ dysfunction and death [4]. Approximately 70% of AL amyloidosis patients have complications with renal involvement, which is often the major cause of morbidity in patients with AL amyloidosis [5]. According to a report from Western countries, AL amyloidosis is the most common type of systemic amyloidosis, with an incidence of 8–10 cases per million person-years, a median age at diagnosis of 63 and a median survival time if left untreated of 12 months [1].
Knowledge about the clinical feature and the patient outcome of AL amyloidosis have mostly been from Western countries, and data in Chinese patients are rarely reported. In the present study, a total of 231 cases of systemic AL amyloidosis patients from the Jinling Hospital amyloidosis registry were enrolled. We analyzed the clinical and follow-up data to reveal the clinical characteristics and risk factors associated with the outcome of patients with AL amyloidosis.
Materials and methods
Patients in the Jinling Hospital amyloidosis registry from 2003 to 2011 were enrolled in this study. All of them are Chinese Han, which is the major ethnic group in Mainland China. This study was approved by the institutional ethical review board of Jinling Hospital. The inclusion criteria included the following: (i) renal biopsy confirmed AL amyloidosis; (ii) biopsy of other sites (such as subcutaneous fat and rectal mucosa) that confirmed AL amyloidosis complicated with renal involvement (urine protein >0.5 g/24 h and/or renal insufficiency) and (iii) follow-up period over 3 months or the patient having reached the endpoint within the 6 months of follow-up.
The clinical data collected included the following information: gender, age at diagnosis, disease duration, clinical symptoms, biopsy location, affected organs, blood pressure, follow-up time, whether special treatment was received (such as chemotherapy or stem cell transplantation) and patient and kidney survival. The baseline blood and urine samples were taken at presentation. The laboratory exams performed included the following: serum creatinine, urea nitrogen, albumin, alkaline phosphatase, hemoglobin, levels of serum free light chains (FLCs), urine protein, N-acetyl-beta-d-amino-glucosidase (NAG), retinol-binding protein (RBP) and hematuria, immunofixation electrophoresis (IFE), liver costal oblique diameter, interventricular septum thickness, left ventricular ejection fraction and bone marrow aspiration and/or biopsy examination.
Definition of related indicators
The follow-up time was defined as the duration from the time of diagnosis to death or the last clinical follow-up. The cutoff point for follow-up was 1 November 2011. The follow-up endpoint was patient death. The secondary endpoint was the onset of end-stage renal disease (ESRD) or dialysis. The starting point of survival time was defined as the date of an established diagnosis of AL amyloidosis. ESRD was defined as the time when the estimated glomerular filtration rate (eGFR) <15 mL/min • 1.73 m2 or at the onset of renal replacement therapy. All patients who died before reaching ESRD were regularly censored. The rate of renal function decline was determined by the slope of eGFR changes. The initiation point of renal survival period was defined as the date of confirmed diagnosis of AL amyloidosis. The mean arterial blood pressure (MAP) was calculated as diastolic pressure +1/3 pulse pressure difference. The criteria of organ involvement were based on international standards. Specialized treatment was defined as patients receiving chemotherapy or autologous stem cell transplantation.
Statistical analysis
The t-test for independent samples or the Mann–Whitney U-test was used to compare the continuous data between groups. The differences between the categorical variables were assessed using Fisher's exact test. Cox proportional hazards were used to calculate the hazard ratios (HRs) for each variable. The P-value reported was two-sided, and P < 0.05 was considered to be statistically significant. All analyses were performed using SPSS software (version 13.0, SPSS, Inc., Chicago, IL, USA).
Results
The epidemiological features
From 2003 to 2011, a total of 269 patients were diagnosed with AL amyloidosis and registered in the Jinling Hospital amyloidosis registry. During this study, 38 patients were lost to follow-up, therefore, a total of 231 cases were included in this study. Among the 231 patients, 153 (66.2%) were male, and 78 (33.8%) were female. The mean age was 56 years: 3.7% of the patients were under 40 years of age, and 7.3% of the patients were over 70 years of age. In addition to the kidney, the most commonly involved organ was the intestinal tract (55.9%), followed by cardiac involvement (46.7%). Hepatic (12.6%) and peripheral nerve involvement (7.7%) were not commonly observed in this group of patients. In terms of affected organs, 25.1% of patients only had renal involvement, 34.6% had two organs involved, 33.3% had three organs involved and 7% of patients had more than three organs involved.
The clinical features
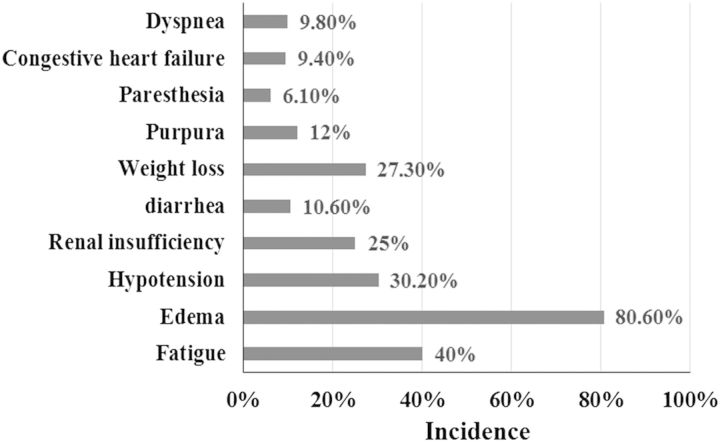
The spectrum of presentations of these patients is diverse. The patients primarily presented with fatigue (40%) and edema (80.6%) at the time of diagnosis followed by hypotension (30.2%) and weight loss (27.3%). At the time of diagnosis, 25% of patients had renal insufficiency. Other uncommon clinical manifestations included purpura (12%), recurrent diarrhea (10.6%), congestive heart failure (9.4%), dyspnea (9.8%) and paresthesia (6.1%) (Figure 1). The diagnosis was mainly based on the renal biopsy. A total of 202 patients in this study underwent renal biopsy, and subcutaneous fat biopsy was performed in 40 patients with a positive rate of 92.5%. Rectal mucosa biopsy was performed in 158 patients with a positive rate of 86.7% (Figure 2).
Fig. 1.
The incidence of symptoms at presentation.
Fig. 2.
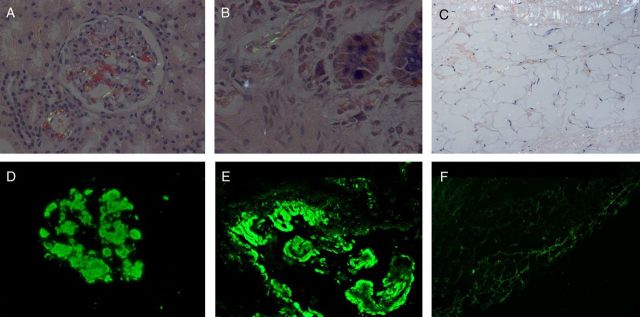
The positive results of Congo red stain and light-chain stain of renal, rectal mucosa and subcutaneous fat. A, A Congo red stain shows apple green birefringence under polarized light of renal tissue. B, The λ light-chain stain of renal tissue is positive, and the stain for κ light chain is negative. C, A Congo red stain shows apple green birefringence under polarized light of rectal mucosa tissue. D, The κ light-chain stain of rectal mucosa tissue is positive, and the stain for λ light chain is negative. E, A Congo red stain shows apple green birefringence under polarized light of subcutaneous fat. F, The λ light-chain stain of subcutaneous fat is positive, and the stain for κ light chain is negative.
The clinical features at diagnostic descriptions are summarized in Table 1. Prior to diagnosis, the median disease course was 7 months (interquartile range, 3–13 months). Nephrotic syndrome (NS) was the most prominent clinical manifestation in these patients, and 147 (63.6%) cases presented with NS at diagnosis. At the time of diagnosis, NAG enzymes and RBP were significantly increased. Renal tubular injury was relatively common in this group of patients. The average creatinine level was 1.21 ± 0.94 mg/dL, and 25% patients had renal dysfunction, 10 patients showed creatinine >4 mg/dL and 75 patients (32.4%) had anemia with an average hemoglobin level of 12.5 ± 2.21 g/dL. The median percentage of bone marrow plasma cell was 2%.
Table 1.
Patient characteristics
| AL (n = 231) | Type of light chain involved |
P-value | ||
|---|---|---|---|---|
| AL-λ (n = 198) | AL-κ (n = 33) | |||
| Male, n (%) | 153 (66.2) | 119 (60.1) | 22 (66.7) | 0.57 |
| Age (years) | 56.02 ± 9.51 | 56.63 ± 9.30 | 52.36 ± 10.05 | 0.02 |
| Organ involvement | ||||
| Renal, n (%) | 231 (100%) | 198 (100%) | 33 (100%) | 1.0 |
| Heart, n (%) | 108 (46.7%) | 91 (46.0%) | 17 (51.5%) | 0.58 |
| Liver, n (%) | 29 (12.6%) | 21 (10.6%) | 8 (24.2%) | 0.04 |
| NS, n (%) | 147 (63.6%) | 121 (61.1%) | 26 (78.8%) | 0.05 |
| Number of involved organ | ||||
| 1 organ, n (%) | 58 (25.1%) | 52 (26.3%) | 6 (18.2%) | 0.39 |
| 2 organs, n (%) | 80 (34.6%) | 71 (35.8%) | 9 (27.3%) | |
| ≥3 organs, n (%) | 93 (40.3%) | 75 (37.9%) | 18 (54.5%) | |
| Positive in serum IFE, n (%) | 157 (67.9%) | 137 (69.2%) | 20 (60.6%) | 0.68 |
| Hb (g/L) | 12.5 ± 2.21 | 12.55 ± 2.11 | 12.16 ± 2.76 | 0.34 |
| MAP (mmHg) | 85.40 ± 14.45 | 84.90 ± 14.54 | 88.43 ± 13.71 | 0.19 |
| Proteinuria (g/24 h) | 4.92 ± 2.92 | 4.66 ± 2.68 | 6.47 ± 3.73 | 0.001 |
| Tubular injury | ||||
| NAG [U/(g Cr)] | 53.8 | 49.7 | 71.4 | 0.03 |
| RBP( mg/L) | 0.58 | 0.49 | 2.37 | 0.001 |
| Serum creatinine (mg/dL) | 1.21 ± 0.94 | 1.14 ± 0.87 | 1.69 ± 1.22 | 0.03 |
| Serum albumin (g/L) | 26.32 ± 6.51 | 26.31 ± 6.47 | 26.33 ± 6.91 | 0.98 |
| Globulin (g/L) | 20.77 ± 6.20 | 20.9 ± 6.33 | 20.0 ± 5.36 | 0.44 |
| Total cholesterol (mmol/L) | 8.46 ± 4.09 | 8.49 ± 4.14 | 8.29 ± 3.82 | 0.79 |
| Bone marrow plasma cells (%) | 2.0 | 2 | 2.5 | 0.16 |
| Alkaline phosphatase (U/L) | 54 | 52 | 63 | 0.08 |
| Cardiovascular events | ||||
| Ejection fraction (%) | 61.88 ± 11.09 | 62.10 ± 10.70 | 60.54 ± 13.31 | 0.46 |
| Interventricular septum (mm) | 11.90 ± 2.63 | 11.75 ± 2.52 | 12.83 ± 3.05 | 0.03 |
The amyloid features
Among all patients, 198 patients were diagnosed with light-chain λ-type deposition (AL-λ) (198/231, 85.7%) and 33 patients had light-chain κ-type deposition (AL-κ) (33/231, 14.3%). The M-proteins noted in patients were primarily λ IgG (36.1%) and λ IgA (18.8%). A total of 32.7% of patients tested M-protein negative. When compared with λ-type AL patients, κ-type patients seem younger at the time of diagnosis (52.36 ± 10.05 versus 56.63 ± 9.30, P = 0.02). Furthermore, the κ-type patients had significantly more urinary protein (6.47 ± 3.73 versus 4.66 ± 2.68, P = 0.001) and more severe renal impairment (1.69 ± 1.22 versus 1.14 ± 0.78, P = 0.03). The NAG enzymes and RBP were also significantly higher in κ-type AL patients, and liver involvement was also more common in κ-type AL patients.
The cause of death
A total of 101 patients (43.7%) died during the follow-up period, and the causes of death are summarized in Table 2. Heart disease remained the primary cause of death, accounting for 34.7% of all the deaths, followed by infection (19.8%) and disease progression (15.8%). Complications associated with dialysis and other treatment (chemotherapy or stem cell transplantation) also contributed to a percentage of deaths. Other causes of deaths included seizure (one case) and lung cancer (one case). Fourteen patients died from unexplained causes.
Table 2.
Causes of death in all patientsa
| AL-λ (n = 198) | AL-κ (n = 33) | Total | |
|---|---|---|---|
| Cardiovascular disease | 29 | 6 | 35 |
| Infection | 17 | 3 | 20 |
| Disease progression | 13 | 3 | 16 |
| Treatment complication | 5 | 1 | 6 |
| Dialysis complication | 4 | 1 | 5 |
| Liver failure | 2 | 1 | 3 |
| Lung cancer | 1 | 0 | 1 |
| Seizure | 0 | 1 | 1 |
| Unknown causes of death | 12 | 2 | 14 |
| Deaths | 83 (41.9%) | 18 (54.5%) | 101 (43.7%) |
aThere is no difference between AL-λ group and AL-κ group.
Patient survival and renal outcome
The median follow-up period was 15.2 months (interquartile range, 7.4–31.2 months), and 101 patients died during the follow-up period. Among them, 30 patients received dialysis before death. A total of 15 patients are currently on dialysis, including 3 patients receiving peritoneal dialysis and 12 patients receiving hemodialysis. The remaining patients survived.
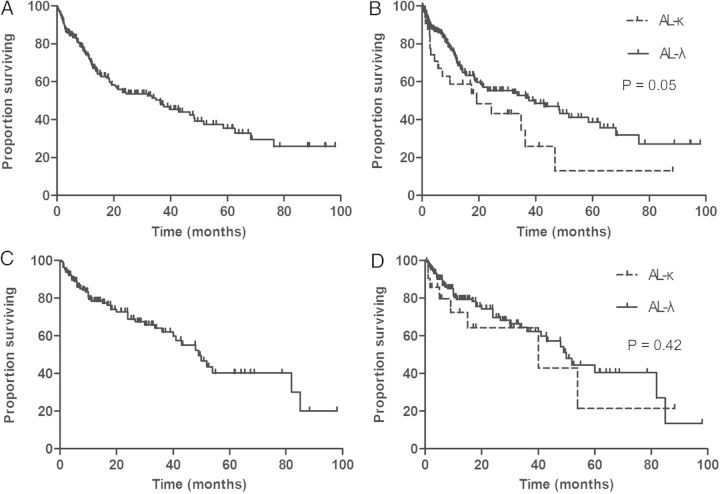
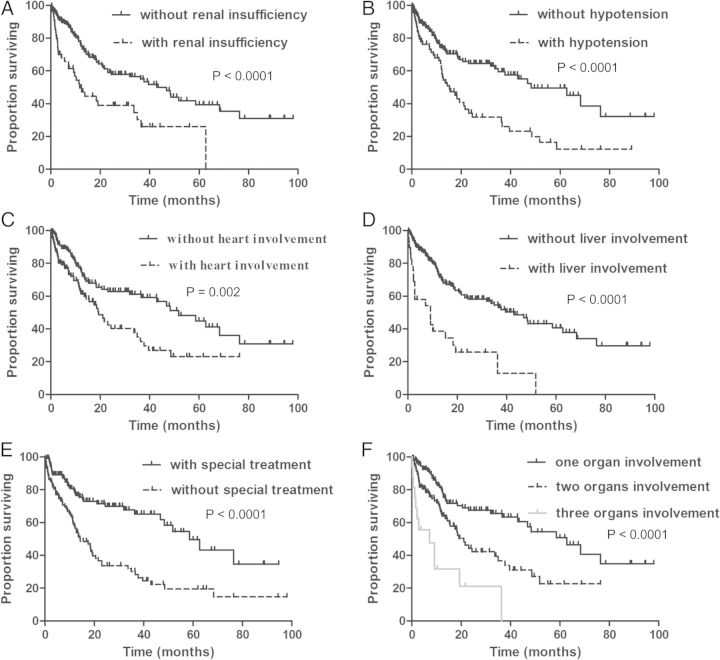
The overall median survival time for patients was 36.3 months (range, 1–98 months). The survival rates at 1, 2, 3 and 5 years were 67, 53, 48 and 35%, respectively (Figure 3A). The median survival was 39.6 months in AL-λ patients and 19.6 months in AL-κ patients (P = 0.05) (Figure 3B). The patients were divided into different subgroups according to the clinical characteristics (Figure 4). The results showed that patient survival time was significantly shorter in patients with renal dysfunction (Figure 4A), hypotension (Figure 4B), cardiac (Figure 4C) or liver involvement (Figure 4D). The median survival was 42.9 months in patients without renal insufficiency and 11.7 months in patients with renal insufficiency (P < 0.0001). The median survival in patients with or without hypotension was 15.2 versus 48.3 months, respectively (P < 0.0001). Similarly, the median survival in patients without heart or liver involvement was 51.8 and 42.9 months, respectively, but the median survival was 19.3 months (P = 0.002) in patients with heart involvement and 9.2 months (P < 0.0001) in patients with liver involvement. However, the survival time in patients receiving special treatment (chemotherapy or stem cell transplantation) was significantly improved (Figure 4E). The median survival was 58.6 months in patients with special treatment and 14.2 months in patients without special treatment (P < 0.0001). In addition, the survival time was significantly shorter in patients with multiple organ involvement (Figure 4F). The median survival was 62.7 months in patients with one organ involvement, 21.1 months in patients with two-organ involvement and 7.1 months in patients with three-organ involvement (P < 0.0001).
Fig. 3.
Overall survival and renal outcome of all patients and between AL-κ and AL-λ. A, Overall survival of all patients. B, Overall survival between AL-κ and AL-λ. C, The renal outcome of all patients. The median renal survival time is 49 months. D, The renal outcome between AL-κ and AL-λ.
Fig. 4.
Kaplan–Meier curves demonstrating differences in overall survival. A, Survival difference between patients with and without renal insufficiency. B, Survival difference between patients with and without hypotension. C, Survival difference between patients with and without heart involvement. D, Survival difference between patients with and without liver involvement. E, Survival difference between patients with and without special treatment. F, Survival difference between patients with different number of organ involvement.
A total of 47 patients received dialysis treatment during the follow-up period, of which 37 patients died, and 10 patients are still alive. Forty-one (87.2%) patients received hemodialysis, and 6 patients received peritoneal dialysis. After excluding the patients who died with normal renal function, the calculated median renal survival time was 49 months (Figure 3C). The 1-, 2-, 3- and 5-year renal survival rates were 78, 69, 62 and 37%, respectively. The median renal survival was 50 months in AL-λ patients and 40 months in AL-κ patients (P = 0.42) (Figure 3D). The patients with eGFR <60 mL/min at diagnosis were analyzed (eGFR was calculated using the Modification of Diet in Renal Disease formula), and the GFR decline rate was 3.44 mL/min/month. The median survival was 18 months in dialysis patients. A total of 36.8% patients died within 1 year of dialysis. The leading causes of death after dialysis were cardiovascular events and infection.
The univariate analysis showed that age, gender, cardiac and liver involvement, hypotension, creatinine levels, proportion of bone marrow plasma cells and receiving special treatment were all closely correlated with disease prognosis. Multivariate analysis showed that age (HR, 1.02; P = 0.042), liver involvement (HR, 2.27; P = 0.002), cardiac involvement (HR, 1.71; P = 0.022) and the proportion of bone marrow plasma cells (HR, 1.08; P = 0.012) were risk factors for patient prognosis. However, special treatment was a protective factor for patient prognosis (Table 3).
Table 3.
Factors associated with the outcome of all patients
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.04 (1.02–1.06) | 0.000 | 1.02 (1.01–1.04) | 0.042 |
| Sex | 1.58 (1.07–2.37) | 0.026 | 1.17 (0.72–1.91) | 0.528 |
| Cardiac involvement | 2.42 (1.26–4.01) | 0.000 | 1.71 (1.08–2.69) | 0.022 |
| Liver involvement | 2.73 (1.73–4.30) | 0.000 | 2.27 (1.34–3.83) | 0.002 |
| Hypotension | 2.08 (1.43–3.02) | 0.000 | 1.09 (0.69–1.71) | 0.698 |
| Serum creatinine | 1.35 (1.19–1.52) | 0.000 | 1.15 (0.96–1.36) | 0.125 |
| The proportion of bone marrow plasma cells | 1.12 (1.07–1.18) | 0.000 | 1.08 (1.04–1.12) | 0.012 |
| Special treatment | 0.41 (0.28–0.60) | 0.000 | 0.39 (0.24–0.58) | 0.000 |
| Disease duration | 0.99 (0.98–1.01) | 0.772 | ||
| Involved serum free light chains | 1.05 (0.99–1.10) | 0.087 | ||
| Proteinuria | 1.03 (0.99–1.13) | 0.057 | ||
| Total cholesterol | 1.01 (0.96–1.06) | 0.730 | ||
| Serum albumin | 0.96 (0.93–1.03) | 0.064 | ||
| Globulin | 0.98 (0.95–1.02) | 0.357 | ||
| Ejection fraction | 0.88 (0.74–1.18) | 0.115 | ||
| Interventricular septum | 1.01 (0.92–1.09) | 0.917 | ||
| Abnormal alkaline phosphatase | 2.13 (0.91–4.17) | 0.075 | ||
| Hemoglobin | 0.96 (0.88–1.05) | 0.332 | ||
| Involved organs ≥2 | 1.32 (0.78–1.89) | 0.233 | ||
The univariate analysis of renal prognosis showed that age, gender, creatinine level, RBP, cardiac involvement, liver involvement and hypotension at diagnosis were all risk factors for ESRD progression. Multivariate analysis showed that the creatinine level and hypotension at diagnosis were independent risk factors for ESRD progression (Table 4).
Table 4.
Factors associated with renal survival of all patients
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.98 (1.11–3.51) | 0.020 | 0.73 (0.37–1.40) | 0.341 |
| Sex | 1.05 (1.02–1.08) | 0.001 | 1.02 (0.99–1.05) | 0.124 |
| Serum creatinine | 2.01 (1.69–2.39) | 0.000 | 1.74 (1.32–2.30) | 0.000 |
| RBP | 1.04 (1.02–1.05) | 0.000 | 1.01 (0.98–1.04) | 0.392 |
| Cardiac involvement | 1.99 (1.16–3.42) | 0.013 | 1.12 (0.61–2.06) | 0.724 |
| Liver involvement | 3.57 (1.82–7.01) | 0.000 | 1.71 (0.81–3.66) | 0.161 |
| Hypotension | 2.02 (1.18–3.48) | 0.011 | 2.01 (1.11–3.63) | 0.020 |
Discussion
AL amyloidosis is a systemic disease that can involve many vital organs. The kidney and heart are the two organs most commonly involved, followed by the liver and peripheral nerves [6]. The patient prognosis is to a large extent determined by the number of organs involved and the severity of the involvement. The patients with severe cardiac involvement have the worst prognosis [7]. In this study, we report for the first time about systemic AL amyloidosis in the Chinese Han population including clinical features and long-term follow-up outcomes.
The spectrum of presentations of AL amyloidosis is diverse, and the presenting symptoms can be very broad and are often mimicked by more common disorders [8]. The clinical features of AL amyloidosis are mainly dependent on the number and the extent of involved organs [9]. For patients with renal involvement, the major clinical manifestations include nephrotic-range proteinuria, acute or chronic renal dysfunction [10]. The overall prognosis in AL amyloidosis patients is poor. The major determinant of outcome in amyloidosis is the extent of cardiac involvement [11]. Outcome is also dependent on the plasma cell clone. The FLC level at diagnosis [12], the number of organs involved [13] and serum uric acid level [14] have all been associated with prognosis.
The clinical features of AL amyloidosis have significant value in early diagnosis. AL amyloidosis frequently occurs in the elderly. The median age of AL amyloidosis is 62 years [15]. In this study, the patient median age was 56 years, 3.7% of the patients were younger than 40 years, and 62.5% of the patients were males, consistent with other reports. A biopsy is necessary for the diagnosis of AL amyloidosis. In this study, a renal biopsy was used as the primary diagnostic method. In addition, skin and rectal mucosa biopsies also had relatively high positive rates of 92.5 and 86.7%, respectively. The results were consistent with those of previous studies by Kaplan et al. [16], indicating that skin and rectal mucosa biopsies were good alternative diagnostic methods in patients with suspected amyloidosis when renal biopsy is not available. The major clinical manifestations of AL amyloidosis were edema (40%) and fatigue and weight loss (35–45%) [17]. In this study, all patients had renal involvement, and 80.6% of the patients had edema, which was most commonly caused by NS. In addition, heart failure and obstructive liver disease can also cause edema.
NS is the main clinical manifestation of renal AL amyloidosis. There is no significant correlation between the extent of amyloid deposition and amount of proteinuria. Even a small amount of amyloid deposition can lead to severe NS [18]. Liver involvement and renal dysfunction seems more common in patients of κ-type in our series, and this result is consistent with previous studies [19]. The most common cause of death for patients with AL amyloidosis was heart disease, and ∼65–75% of the patients died of cardiac amyloidosis-related complications. We also performed a correlation analysis of related factors in cardiac involvement. The univariate and multivariate analyses both showed that cardiac involvement was a risk factor for poor patient prognosis. The median survival time in patients with cardiac involvement was significantly reduced.
Patients with AL amyloidosis can have different prognoses. The multifactor analysis of this study identified age, liver and cardiac involvement as independent prognostic risk factors. Liver involvement, though not as common as heart and kidney, had a major influence on patient prognosis. Patients with elevated bilirubin >2 mg/dL only had a median survival time of 1–2 months [20]. In a group with 98 cases of AL amyloidosis with liver involvement, the clinical analysis showed that the median survival time was only 8.5 months [20]. Most patients with liver involvement in this study also had cardiac involvement; therefore, these patients had an even worse prognosis with a median survival time of only 9.2 months. Previous studies have shown that the proportion of bone marrow plasma cells and the plasma cell κ/λ ratio were also associated with patient prognosis [21]. Our study found that the proportion of bone marrow plasma cells had no influence on prognosis, which is not consistent with previous results.
The kidney is the most common organ involved in AL amyloidosis, which often results in irreversible renal damage and ESRD. The Mayo Clinic has reported the long-term follow-up results on a group of 145 AL amyloidosis patients with renal involvement showing that the 24 h urinary protein and creatinine levels at diagnosis were prediction factors for renal involvement and the median survival time from the day of dialysis was <1 year [22]. Montseny et al. [23] showed that the median time from AL amyloidosis diagnosis to dialysis was 15 months and that the median survival time was 24 months. The above studies all showed that after the AL amyloidosis diagnosis, patients progressed to ESRD, the prognosis deteriorated and the median survival time was significantly reduced. The conclusion of this study was consistent with that of other studies. The multivariate analysis also showed that the creatinine levels and hypotension at diagnosis were independent risk factors for progression to ESRD.
Compared with the available data from Western countries, patients with renal AL amyloidosis in our center were younger at the time of diagnosis. In addition, liver and peripheral nervous system involvement were less common in our series. However, the clinical manifestations were similar to previous results, and the median survival of our patients was equal with the data from Europe [24]. Moreover, the mean rate of decline in renal function was much faster in our patients.
The limitations of this study must be recognized. First, as a retrospective study, we excluded the patients who were lost to follow-up or had incomplete clinical data, which may lead to a prognosis that was overestimated. Second, less than one-fourth of the patients in this study received dialysis treatment, which may be due to the short follow-up time. In addition, some patients died before the progression to ESRD, which can also lead to low dialysis rates. Finally, cardiac biomarkers were not detected in some of the patients; therefore, we cannot analyze the relationship between the cardiac biomarkers and prognosis.
Conclusions
AL amyloidosis is a multisystem disease with diverse clinical presentations and poor prognosis. To our knowledge, this is the first report on the prognosis of Chinese AL amyloidosis patients. The main clinical manifestations of these patients were fatigue, edema and hypotension, and NS was observed in most patients with renal involvement. The median survival time of our cohort was 36.3 months, and the 1-, 2-, 3- and 5-year cumulative survival rates were 67, 53, 48 and 35%, respectively. The long-term prognosis in such patients was poor and was even worse in those with cardiac, and/or liver involvement, hypotension and renal dysfunction. However, special treatments can significantly improve the survival rate in those patients. The prognosis of the kidney was also poor, with faster renal deterioration and a shorter survival time in patients on dialysis. High creatinine levels and hypotension at diagnosis are the independent predictors of an unfavorable renal outcome.
Conflict of interest statement. None declared.
References
- 1.Cohen AD, Comenzo RL. Systemic light-chain amyloidosis: advances in diagnosis, prognosis, and therapy. Hematology Am Soc Hematol Educ Program. 2010;2010:287–294. doi: 10.1182/asheducation-2010.1.287. [DOI] [PubMed] [Google Scholar]
- 2.Gertz MA, Lacy MQ, Dispenzieri A, et al. Amyloidosis. Best Pract Res Clin Haematol. 2005;18:709–727. doi: 10.1016/j.beha.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 3.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- 4.Comenzo RL. Amyloidosis. Curr Treat Options Oncol. 2006;7:225–236. doi: 10.1007/s11864-006-0015-8. [DOI] [PubMed] [Google Scholar]
- 5.Dember LM. Amyloidosis-associated kidney disease. J Am Soc Nephrol. 2006;17:3458–3471. doi: 10.1681/ASN.2006050460. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol. 1995;32:45–59. [PubMed] [Google Scholar]
- 7.Falk RH. Cardiac amyloidosis: a treatable disease, often overlooked. Circulation. 2011;124:1079–1085. doi: 10.1161/CIRCULATIONAHA.110.010447. [DOI] [PubMed] [Google Scholar]
- 8.Gertz MA. Immunoglobulin light chain amyloidosis: 2013 update on diagnosis, prognosis, and treatment. Am J Hematol. 2013;88:416–425. doi: 10.1002/ajh.23400. [DOI] [PubMed] [Google Scholar]
- 9.Pinney JH, Hawkins PN. Amyloidosis. Ann Clin Biochem. 2012;49(Pt 3):229–241. doi: 10.1258/acb.2011.011225. [DOI] [PubMed] [Google Scholar]
- 10.Merlini G, Seldin DC, Gertz MA. Amyloidosis: pathogenesis and new therapeutic options. J Clin Oncol. 2011;29:1924–1933. doi: 10.1200/JCO.2010.32.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastritis E, Dimopoulos MA. Prognosis and risk assessment in AL amyloidosis—state of the art. Amyloid. 2011;18(Suppl 1)):89–91. doi: 10.3109/13506129.2011.574354032. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Dispenzieri A, Katzmann JA, et al. Serum immunoglobulin free light-chain measurement in primary amyloidosis: prognostic value and correlations with clinical features. Blood. 2010;116:5126–5129. doi: 10.1182/blood-2010-06-290668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gertz MA, Zeldenrust SR. Treatment of immunoglobulin light chain amyloidosis. Curr Hematol Malig Rep. 2009;4:91–98. doi: 10.1007/s11899-009-0013-6. [DOI] [PubMed] [Google Scholar]
- 14.Kumar S, Dispenzieri A, Lacy MQ, et al. Serum uric acid: novel prognostic factor in primary systemic amyloidosis. Mayo Clin Proc. 2008;83:297–303. doi: 10.4065/83.3.297. [DOI] [PubMed] [Google Scholar]
- 15.Gertz MA, Lacy MQ, Dispenzieri A. Immunoglobulin light chain amyloidosis and the kidney. Kidney Int. 2002;61:1–9. doi: 10.1046/j.1523-1755.2002.00085.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan B, Vidal R, Kumar A, et al. Immunochemical microanalysis of amyloid proteins in fine-needle aspirates of abdominal fat. Am J Clin Pathol. 1999;112:403–407. doi: 10.1093/ajcp/112.3.403. [DOI] [PubMed] [Google Scholar]
- 17.Dispenzieri A, Merlini G, Comenzo RL. Amyloidosis 2008 BMT Tandem Meetings (February 13–17, San Diego) Biol Blood Marrow Transplant. 2008;14(Suppl 1):6–11. doi: 10.1016/j.bbmt.2007.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Hetzel GR, Uhlig K, Mondry A, et al. AL-amyloidosis of the kidney initially presenting as minimal change glomerulonephritis. Am J Kidney Dis. 2000;36:630–635. doi: 10.1053/ajkd.2000.16205. [DOI] [PubMed] [Google Scholar]
- 19.Russo P, Palladini G, Foli A, et al. Liver involvement as the hallmark of aggressive disease in light chain amyloidosis: distinctive clinical features and role of light chain type in 225 patients. Amyloid. 2011;18(Suppl 1)):92–93. doi: 10.3109/13506129.2011.574354033. [DOI] [PubMed] [Google Scholar]
- 20.Park MA, Mueller PS, Kyle RA, et al. Primary (AL) hepatic amyloidosis: clinical features and natural history in 98 patients. Medicine (Baltimore) 2003;82:291–298. doi: 10.1097/01.md.0000091183.93122.c7. [DOI] [PubMed] [Google Scholar]
- 21.Perfetti V, Colli Vignarelli M, Anesi E, et al. The degrees of plasma cell clonality and marrow infiltration adversely influence the prognosis of AL amyloidosis patients. Haematologica. 1999;84:218–221. [PubMed] [Google Scholar]
- 22.Gertz MA, Leung N, Lacy MQ, et al. Clinical outcome of immunoglobulin light chain amyloidosis affecting the kidney. Nephrol Dial Transplant. 2009;24:3132–3137. doi: 10.1093/ndt/gfp201. [DOI] [PubMed] [Google Scholar]
- 23.Montseny JJ, Kleinknecht D, Meyrier A, et al. Long-term outcome according to renal histological lesions in 118 patients with monoclonal gammopathies. Nephrol Dial Transplant. 1998;13:1438–1445. doi: 10.1093/ndt/13.6.1438. [DOI] [PubMed] [Google Scholar]
- 24.Pinney JH, Lachmann HJ, Bansi L, et al. Outcome in renal Al amyloidosis after chemotherapy. J Clin Oncol. 2011;29:674–681. doi: 10.1200/JCO.2010.30.5235. [DOI] [PubMed] [Google Scholar]