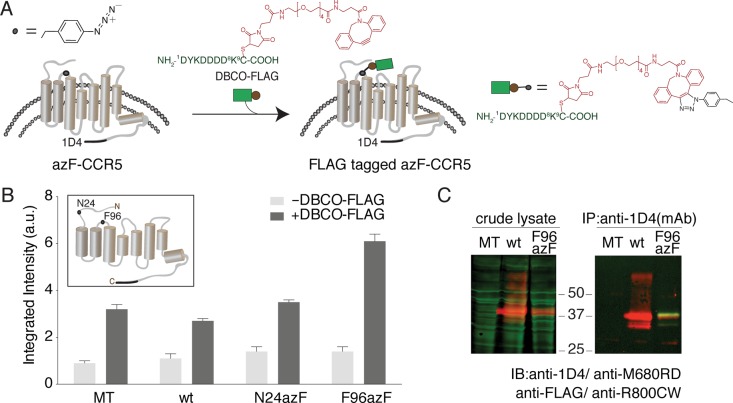
Figure 1.
Fluorophore-linked immunoassay for detecting azF-CCR site-specifically peptide epitope modified by SpAAC. (A) Scheme showing the in-culture labeling of the GPCR, CCR5, the uaa azF genetically encoded at a target site. SpAAC reaction is used to site-specifically attach the FLAG peptide epitope (green) conjugated to a DBCO (red) via a maleimide cross-linker (DBCO-FLAG). (B) Representative results of the on-cell ISA for measuring the FLAG signal with or without DBCO. HEK293T cells expressing N24azF or F96azF CCR5 variants were treated with 100 μM DBCO-FLAG (dark gray bars), and data for the untreated cells are also shown (light gray bars). Wild-type CCR5 (wt) and mock-transfected (MT) controls were tested under the same condition. Error bars represent the standard error of the mean of triplicate measurements. (C) Western blot analysis of labeled and purified azF-CCR5. In the left panel, the crude lysate sample was probed simultaneously with anti-FLAG polyclonal antibody and 1D4 monoclonal antibody to detect the extent of FLAG peptide epitope tagging (green) and full-length receptor expression (red), respectively. The receptor is visualized in the red channel and separates at 37 kDa. The right panel shows full-length CCR5 immunoaffinity purified with the C-terminal 1D4 engineered tag. The purified receptor separates at 37 kDa (red) as detected with the anti-1D4 antibody against the C-terminal 1D4 epitope tag, and the FLAG tag is visualized with the anti-FLAG pAb (green).