Abstract
This study evaluated whether exposure to maternal pre- or postnatal depression or anxiety symptoms predicted psychopathology in adolescent offspring. Growth mixture modeling was used to identify trajectories of pre- and postnatal depression and anxiety symptoms in 577 women of low socioeconomic status selected from a prenatal clinic. Logistic regression models indicated that maternal pre- and postnatal depression trajectory exposure was not associated with offspring major depression, anxiety, or conduct disorder but exposure to the high depression trajectory was associated with lower anxiety symptoms in males. Exposure to medium and high pre- and postnatal anxiety was associated with the risk of conduct disorder among offspring. Male offspring exposed to medium and high pre- and postnatal anxiety had higher odds of conduct disorder than males with low exposure levels. Females exposed to medium or high pre- and postnatal anxiety were less likely to meet conduct disorder criteria than females with lower exposure. To the best of our knowledge, this is the first study to examine the effect of pre- and postnatal anxiety trajectories on the risk of conduct disorder in offspring. These results suggest new directions for investigating the etiology of conduct disorder with a novel target for intervention.
Community-based prevalence studies have demonstrated that up to 25% of children are exposed to elevated levels of pre- or postnatal depressive symptoms and a similar proportion are exposed to pre- and postnatal anxiety symptoms (Heron, O'Connor, Evans, Golding, & Glover, 2004). These early-life exposures may, in turn, contribute to children’s risk of developing psychopathology later in life through a complex interplay of underlying genetic risk, biochemical alterations, and environmental factors.
The fetal programming hypothesis asserts that during the prenatal period, exposure to high maternal anxiety and depression symptoms may lead to elevated levels of maternal stress hormones, particularly cortisol, crossing the placental barrier and affecting fetal development (Austin, Leader, & Reilly, 2005; de Weerth & Buitelaar, 2005; Fumagalli, Molteni, Racagni, & Riva, 2007; O'Connor, Heron, Golding, & Glover, 2003; Van den Bergh & Marcoen, 2004). In the postnatal period, infants and mothers form emotional attachment patterns that are the foundation for relationships throughout their lives (McMahon, Barnett, Kowalenko, & Tennant, 2006; Pastor, 1981). Exposure to maternal depression and anxiety symptoms during this period may disrupt the development of healthy attachment patterns (Davis et al., 2004; McMahon, et al., 2006) and negative attachment styles, have been associated with a number of psychiatric illnesses, including personality disorders (Westen, Nakash, Thomas, & Bradley, 2006), substance abuse (Schindler, Thomasius, Sack, Gemeinhardt, & Kustner, 2007), depression (West & George, 2002), and eating disorders (Ward, Ramsay, Turnbull, Benedettini, & Treasure, 2000; Ward et al., 2001), as well as nonspecific psychopathology in children and adolescents (Brown & Wright, 2003; Fergusson, Woodward, & Horwood, 2000; McCartney, Owen, Booth, Clarke-Stewart, & Vandell, 2004; Yoo, Kim, Shin, Cho, & Hong, 2006). Both biochemical and environmental risk factors may interact with underlying genetic influences, which can increase the cumulative risk for offspring psychopathology (Pemberton et al., 2010).
Numerous studies have linked prenatal anxiety and depression exposure to adverse child outcomes, including fussiness and difficult temperament in infants and children (Austin, Hadzi-Pavlovic, Leader, Saint, & Parker, 2005; Coplan, O'Neil, & Arbeau, 2005; Davis, et al., 2004; Huizink, de Medina, Mulder, Visser, & Buitelaar, 2002; Papousek & von Hofacker, 1998). The Avon Longitudinal Study of Parents and Children (ALSPAC), a large prospective cohort study in England, reported that in 4-year-olds (O'Connor, Heron, Golding, et al., 2002), prenatal anxiety symptoms (at 18 or 32 weeks of pregnancy) were associated with more emotional problems in girls and boys, more conduct problems in girls, and more hyperactivity/attention problems in boys, controlling for maternal postnatal anxiety and depression (O'Connor, Heron, Golding, Beveridge, & Glover, 2002). At the 6.75-year assessment of this cohort, prenatal maternal anxiety predicted conduct and hyperactivity/attention problems in both boys and girls (O'Connor, Heron, Golding, & Glover, 2003).
Similarly, in a community-based sample of physically healthy mothers and their 8- and 9-year-old children, Luoma and colleagues (2001) found that elevated rates of depressive symptoms in the third trimester were associated with higher total problem scores on the Child Behavior Checklist [CBCL, (Achenbach & Edelbrock, 1983)], specifically, more externalizing disorders, independent of postnatal and later maternal depression symptoms. In a recent comprehensive study examining genetic, prenatal, and environmental risk factors on the development of externalizing symptoms from infancy to toddlerhood, Pemberton and colleagues (2010) found that prenatal depression symptoms among biological mothers were indirectly associated with externalizing behaviors through riskier pregnancies (e.g., lack of prenatal care).
Leech et al. (2006) examined a number of predictors of high depression and anxiety symptoms in the 10-year-old children in a sample of generally healthy women of low socioeconomic status (SES) who were recruited during pregnancy. Consistent with the previous findings, higher prenatal depression symptom exposure was associated with the children’s combined depression and anxiety symptoms at age 10, controlling for the mother’s depression and anxiety at 18 months, 3 years, and 6 years postnatally.
The effects of exposure to prenatal depressive and anxiety symptoms on offspring psychiatric diagnoses have received less attention. In a retrospective study of high school students and their parents (Allen, Lewinsohn, & Seeley, 1998), diagnoses of Major Depressive Disorder (MDD), Anxiety Disorders, and Disruptive Behavior Disorders (DBD) were obtained using the Schedule for Affective Disorders and Schizophrenia for School-Age Children [K-SADS; (Orvaschel, Puig-Antich, Chambers, Tabrizi, & Johnson, 1982)]. Combined maternal anxiety and depression symptoms during pregnancy predicted the occurrence of MDD and DBD in the youth, but not anxiety disorders. In a prospective study of a community-based sample of pregnant women, Pawlby et al. (2009) found a four-fold increase in MDD in 16-year-olds exposed to maternal MDD in the second or third trimester but this association became non-significant when the number of subsequent maternal depressive episodes was included in the model.
Exposure to postnatal maternal symptoms and outcomes in the offspring has received more attention than maternal symptoms in the prenatal period, particularly with regard to postnatal depression symptoms. Numerous studies have examined postnatal depression effects on children’s cognitive (Beck, 1998; Sutter-Dallay et al., 2010), behavioral (Avan, Richter, Ramchandani, Norris, & Stein, 2010; Barker, Copeland, Maughan, Jaffee, & Uher, 2012; Beck, 1998; Grace, Evindar, & Stewart, 2003; Pemberton et al., 2010), and emotional problems (Araya et al., 2009; Barker et al., 2012; Verbeek et al., 2011). In a meta-analysis, Beck (1998) summarized nine studies and found a small effect size for the relation between postnatal depression and poorer cognitive development and behavioral problems. In a systematic review, Grace, Evindar, and Stewart (2003) also reported an effect of postnatal depression on child behavioral problems, although they warn that their findings may not be specific to exposure during the immediate postnatal period but rather might reflect the effects of chronic maternal depression on child behavior. Moreover, the study by Pemberton et al. (2010) examined exposure to depression symptoms in adoptive parents in relation to the development of externalizing symptoms in toddlers and found that adoptive father’s depression symptoms at 9 months postpartum and adoptive mother’s depressive symptoms across 9, 18, and 27 months (measured as a single factor) were associated with externalizing behaviors, even after controlling for biological mother’s depression symptoms.
Fewer studies have examined the effect of postnatal anxiety exposure on general psychiatric outcomes. Barnett and colleagues (Barnett, Schaafsma, Guzman, & Parker, 1991) reported that 5-year-old children of community-sampled mothers with high postnatal anxiety symptoms were less active than the children exposed to lower levels of anxiety. Additionally, boys exposed to high levels of maternal anxiety symptoms were less socially competent and more immature, delinquent, and schizoid than their unexposed counterparts. Similarly, the ALSPAC study found that the risk of emotional problems was 50% greater in 4-year-olds exposed to higher postnatal anxiety symptoms, and exposed girls were at a 45% increased risk of conduct problems, independent of prenatal depression and anxiety symptoms and postnatal depressive symptoms (O'Connor, Heron, Golding, et al., 2002). At age 6, the risk for emotional problems in boys was increased by 60%, but there was no longer an elevated risk for emotional problems in girls (O'Connor, et al., 2003). However, the rate of conduct problems in exposed girls at 6 years was double the rate in unexposed girls.
A few studies have emerged recently examining postnatal depression symptom exposure and psychiatric disorders in offspring. Murray, et al. (2011) prospectively assessed offspring of clinically depressed and non-depressed mothers from infancy through 16 years postpartum and found that the 16 year olds of postnatally depressed mothers were more likely to be depressed. Similarly, a study by Pawlby and colleagues (2008) found that 11-year-old offspring of mothers with postnatal clinical depression were four times more likely to meet criteria for a psychiatric diagnosis than offspring of non-depressed controls. No studies were identified that specifically examined anxiety exposure in the postnatal period and the risk for psychiatric disorders in adolescents.
These studies support the hypothesis that exposure to pre- and postnatal maternal depression and anxiety symptoms may increase the risk for subsequent mental health symptoms in children, including mental health symptoms reaching clinically significant thresholds (Allen, Lewinsohn, & Seeley, 1998; Murray, et al., 2011; Pawlby, et al., 2008). Moreover, this pattern continues into pre-adolescence and adolescence (Allen, Lewinsohn, & Seeley, 1998; Murray, et al., 2011; Pawlby, et al., 2008). However, several research challenges exist. First, women with postnatal symptoms are also usually symptomatic during pregnancy, making it difficult to isolate the effects of prenatal exposure from postnatal exposure without very large samples (Fergusson, Horwood, & Thorpe, 1996; Heron, et al., 2004). Studies that have examined postnatal depression effects typically did not consider the impact of prenatal depression, with one notable exception. Hay and colleagues (2008) examined the effect of prenatal, postnatal, and later maternal depression and anxiety symptoms on offspring IQ, emotional disorders, and disruptive behavior disorders. They found, after controlling for antenatal and later depression, that postpartum (within 3 months of birth) depression was associated with lower IQ but not psychopathology in the children. Psychopathology was associated with exposure to later (post-postnatal) maternal depression only, once prenatal and postnatal exposures were controlled for. However, attempts to isolate the relative effects of pre- and postnatal symptoms may not represent the true risk to exposed children. If most children who are exposed to prenatal symptoms also experience postnatal exposure, then the combined effects would represent the typical exposure and attempts to control for one period or the other in a regression framework would obscure the individual level associations. These challenges lead to the question of how best to categorize the maternal symptom exposures in order to evaluate their effects on the psychiatric health of their offspring.
This study attempted to address this question by utilizing trajectory analysis to determine whether exposure to maternal patterns of pre- and postnatal depression and anxiety symptoms increases the risk of major depressive disorder (MDD), anxiety disorder, or conduct disorder (CD) in offspring. Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) syndromal disorders require individuals to meet or exceed pre-defined thresholds for impairment in functioning and cumulative symptom burden (American Psychiatric Association, 2000) and thus far have been understudied. In addition, we examined depression, anxiety, and conduct symptoms to evaluate the consistency of findings beyond syndromal diagnostic criteria.
The study consists of two parts. In the first section, we modeled the most common pre- and postnatal depression and anxiety patterns in women and examined the correlates of mother’s pre- and postnatal depression and anxiety trajectory group memberships. In the second section, we examined the association between pre- and postnatal depression and anxiety trajectory group exposures and the offspring’s risk of developing MDD, anxiety disorders, and CD by age 16, as well as symptom levels for each type of disorder. Several studies have identified increased risk and earlier onset for psychiatric disorders, particularly depression, among children exposed to parental depression and anxiety in childhood, suggesting age 16 is sufficient for detecting disorders that often have an older average age of onset (Klein, Lewinsohn, Rohde, Seeley, & Olino, 2005; Weissman et al., 1987; Weissman, Leckman, Merikangas, Gammon, & Prusoff, 1984). Based upon the previous research, we hypothesized that maternal pre- and postnatal depression would be associated with depression and anxiety disorders in offspring, and maternal pre-natal anxiety exposure would be associated with CD in boys and depression and anxiety disorders in girls.
Methods
Participants and Procedures
Data for this analysis came from the Maternal Health Practices and Child Development Project (MHPCD, PI: Nancy L. Day, NIDA R01 DA03874, NIAAA R01 AA06666) and the Childhood Abuse as a Predictor of Adolescent Alcohol Project (PI: Cynthia A. Larkby, NIAAA K01 AA00312). From 1982 to 1985, 1,360 adult, English-speaking women were contacted through a hospital-based prenatal clinic during their fourth or fifth month of pregnancy. Women were interviewed as part of two longitudinal studies on the effects of prenatal alcohol and prenatal marijuana exposure on offspring development. A stratified sampling design with replacement was used to ensure a distribution of substance use from abstinence to heavy use. The alcohol cohort included all women who drank on average at least three alcoholic drinks per week and a random selection of those who drank less or abstained. The marijuana cohort included all women who smoked two or more joints per month and a random sample of those who used less. Both cohorts were evaluated simultaneously, with the same protocol and instruments, and are combined in these analyses to create a sample of 829 women. By delivery, this number was reduced to 763; 21 had moved, 16 were missed, 8 refused the delivery assessment, 2 had multiple births, 18 infants died, and 1 infant was placed for adoption.
The MHPCD project included 11 assessments: 1st trimester baseline (assessed retrospectively at the 4th or 5th month of pregnancy); 2nd trimester (assessed at the 7th prenatal month); delivery; and when the offspring were 8 and 18 months, 3, 6, 10, 14, and 16 years of age. Assessments for a 22-year follow-up have been completed but those data were not available at the time of these analyses. Follow-up rates for each phase subsequent to birth ranged between 76% and 88% with a mean of 82%. Written consent was obtained from all participants and the study was conducted in accordance with the University of Pittsburgh’s Institutional Review Board and Magee-Womens Hospital’s Research Review and Human Experimentation Committee.
The outcomes of interest for these analyses were psychiatric diagnoses and associated symptom levels in the offspring. Therefore, only mother-child pairs who had completed the child psychiatric assessment at the 16-year follow-up were included in this analysis. Moreover, women missing more than two of the first five assessments (those used in the exposure assessment) were also excluded (along with their offspring) from analyses. This left 577 mother-child pairs (76% of the birth cohort at delivery) who had completed the child psychiatric assessments at the age 16 assessment and had three or more exposure assessments completed. Compared to women included in the analyses, women excluded due to missing data were more likely (p<.05) to be white, 58% of excluded women were white vs. 45% of included women. Offspring who were excluded were more likely to be male (58% vs. 48%) and were less likely to have been prenatally exposed to marijuana (38.5% vs. 30%) than the non-excluded. There were no differences among excluded and non-excluded women and offspring with regard to the other covariates examined, including depression and anxiety scores.
Measures
Maternal symptom trajectories
Exposure data for these analyses came from the first three assessments (1st, 2nd, and 3rd trimesters), collectively referred to as the prenatal period for the rest of this report, and the first two post-delivery assessments (8 and 18 months), referred to as the postnatal period. Pre- and postnatal depression symptoms were measured in the prenatal and postnatal periods using the Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977). The CES-D is a 20-item self-report scale with a 4-point Likert response scale measuring frequency of depressive symptoms. Scores range from 0 to 60 with higher scores indicating more symptoms. The CES-D is widely used, including in studies of pregnant women (Beeghly et al., 2002; Kuo et al., 2004), and has strong psychometric properties in clinical and general populations [Cronbach’s α = .85 and 0.9, respectively; (Boyd, Weissman, Thompson, & Myers, 1982; Radloff, 1977)]. The baseline Cronbach’s α for the CES-D in the MHPCD sample was .86.
Pre- and postnatal anxiety exposure from pregnancy through the postnatal period was assessed using the self-report trait scale of Spielberger’s State-Trait Anxiety Personality Inventory (STPI) (Speilberger, 1979), which is similar to the short-form trait scale of Spielberger’s State-Trait Anxiety Inventory (STAI) (Speilberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). Trait anxiety (T-anxiety) assesses a person’s propensity to react to threatening stimuli with anxiety (Ree, 2008). Scores ranged from 10 to 40, with higher scores indicating higher anxiety levels. The Cronbach’s α at the baseline assessment in the MHPCD sample was .87.
Offspring mental health outcomes
At age 16, the computerized Diagnostic Interview Schedule [DIS-IV; (Robins et al., 2000)] was used to assess self-reported psychiatric illness according to the DSM-IV (American Psychiatric Association., 2000). The DIS-IV is a well-established diagnostic interview that uses the DSM-IV diagnostic criteria (Dascalu, Compton, Horton, & Cottler, 2003; Horton, Compton, & Cottler, 1998). MDD and CD were the most prevalent diagnoses in the offspring by age 16, with sufficient sample size for analysis. Three anxiety disorders were assessed (post-traumatic stress disorder [PTSD], separation anxiety, and generalized anxiety disorder [GAD]) and combined into a single outcome variable (“anxiety”) in order to have a large enough sample size to permit analysis. All analyses used lifetime diagnoses as based on the adolescent's self-report at age 16. Each disorder was calculated separately for each set of analyses. For example, if an adolescent met criteria for MDD, then he or she was coded as having MMD and adolescents who did not meet criteria for MDD were coded as not having MDD, regardless of the presence of other disorders. Thus, the comparison group could include offspring who met criteria for any disorder other than the disorder being examined.
For the offspring symptom level analyses, symptom counts for the MDD and CD DIS-IV diagnostic modules were calculated. The use of a combined category of DIS-IV anxiety disorders precluded the use of symptom counts from the anxiety disorders, as symptoms might then be “double counted”. Therefore, anxiety symptoms were measured using The Revised Children's Manifest Anxiety Scale (RCMAS; Reynolds & Richmond, 1978). The RCMAS is a 37-item self-report measure assessing anxiety symptoms in children and adolescents and has demonstrated reliability and validity for use in children ages 6 to 19.
Statistical Analysis
Modeling Covariates
The two sets of analyses presented in this paper included different covariates that were determined based upon previous literature on pre- and postnatal depression, pre- and postnatal anxiety, and adolescent psychiatric illness. Part One analyses examined the correlates of the pre- and postnatal depression and anxiety symptom trajectories. These trajectories included symptoms measured during the prenatal and postnatal phases. In the Part Two analyses, we explored confounding in the relations between maternal symptom trajectories and offspring psychiatric outcomes and considered covariates from all of the assessment periods (prenatal, postnatal, and 3 years postpartum or later).
For the Part One analyses, the potential correlates of pre- and postnatal depression and anxiety trajectory group membership included demographics, social support, obstetrical complications, and maternal drug use. Baseline demographic measures included: maternal age (years), race (Caucasian, African American), number of prior pregnancies (0, 1, 2 or more), marital status (single, married), education (years), monthly family income, and maternal employment (employed/student or unemployed). First trimester perceived social support was assessed using questions developed for this study based upon measures from the Human Population Laboratory (Berkman & Syme, 1979). The distribution of social support was severely skewed so this variable was dichotomized as low/high support based on the distribution. Pregnancy, labor, and delivery complications were assessed at delivery using common obstetrical complication schemes (Hobel, Hyvarinen, Okada, & Oh, 1973; Littman & Parmelee, 1978; Zax, Sameroff, & Babigian, 1977) and were extracted by chart review at delivery by study nurse clinicians.
Maternal drug use was assessed using measures specifically designed for this study (Day & Robles, 1989; Robles & Day, 1990). Substances assessed included alcohol, tobacco cigarettes, marijuana, cocaine, and other illicit drugs. For Part One analyses, use for the year prior to pregnancy was included as a covariate for all substances except cigarette use, for which pre-pregnancy data were not collected. Both quantity and frequency of use were ascertained and converted into average daily drinks and joints for alcohol and marijuana, respectively. Cocaine and other illicit drugs were used as dichotomous variables. Cigarette use was categorized for this analysis into less than one, 1 – 19, and 20 or more cigarettes per day, based on the baseline distribution.
Part Two covariates included demographics, obstetrical complications, offspring custody status, maternal drug use, and maternal depression and anxiety symptoms after the postnatal period (between 3 and 16 years). Demographics included maternal age, race, number of prior births, marital status, and education; monthly family income (baseline and averaged across all assessments), baseline maternal employment, and offspring sex. Obstetrical complications included pregnancy, labor, and delivery complications. Offspring custody status was categorized as either consistently with the mother at all assessments or inconsistently with the mother.
Prenatal exposure was categorized into 4 groups: 1) women who abstained from all alcohol and drugs while pregnant; 2) those who used in the first trimester only; 3) those who used in the first and second trimesters and then stopped; and 4) those who used in all three trimesters. Since the postnatal period consisted of only two assessments (8 and 18 months), the average use was used to represent alcohol, marijuana, and tobacco consumption. Frequency of cocaine and other drug use was low, and therefore exposure to these substances was combined for all Part Two analyses and was coded using the trimester categorization defined above for the prenatal period and as any versus none for the postnatal period. Bivariate analyses suggested that the later drug use exposure variables could be averaged across the later postpartum assessments (3 years and beyond) for alcohol and marijuana, and dichotomized into any versus no use over that period for other illicit drugs. The extremely high correlation of cigarette use in each time period led us to only consider prenatal and postnatal cigarette use.
To determine the best method of evaluating post-postnatal (3 to 16 years) maternal depression and anxiety symptoms, we examined the association of maternal symptoms at each follow-up period with the three main outcomes (MDD, Anxiety, and CD diagnoses), as well as the average maternal symptoms across offspring ages 3 through 16. Only maternal symptoms in the later assessments, age 14 and 16, were associated with offspring diagnoses by age 16. Therefore, the average of those two assessments was treated as a potential confounder.
Part One analyses: Modeling maternal symptom trajectories
Trajectory analysis was performed using the Growth Mixture Modeling (GMM) function of Mplus (Muthén & Muthén, 1998–2007) that enables identification of subgroups of individuals exhibiting similar patterns of symptoms from among the total sample. Two sets of trajectories were examined representing pre- and postnatal depression and anxiety symptom patterns from the first trimester through 18 months postnatally.
Identification of symptom trajectories began with identifying the optimal number of trajectory groups for each symptom set by consecutively modeling an increasing number of groups until non-convergence was reached. At that point, fit criteria, including the sample size adjusted Bayesian Information Criterion (BIC), the Akaike Information Criteria (AIC), entropy, and the Lo, Mendell, and Rubin (2001) statistic, were used to evaluate model fit. In the case of multiple models fitting equally, the most parsimonious model was selected (Bauer & Curran, 2003). After the number of groups was determined, the shape of each trajectory was examined. To determine trajectory shape, parameters from a linear through a cubic growth curve were tested for significant contribution to the model. Once the trajectory models were established, participants were classified into their most likely trajectory based upon the posterior probability.
Logistic and multinomial regression analyses were used to examine correlates of pre- and postnatal depression and anxiety trajectory group membership. Correlates were tested in bivariate analyses first and were retained in the final model if the bivariate association was significant at the p<.1 level. The broader inclusion level of p<.1 was chosen because the cut point of .05 may exclude variables not strongly associated with the outcome but still able to confound the relationship of other associations within the model.
Part Two analyses: Modeling offspring mental health outcomes
Six logistic regression models were created to examine each exposure and outcome combination: pre- and postnatal depression with offspring MDD, offspring anxiety (combined PTSD, GAD, and separation anxiety), and offspring CD, and pre- and postnatal anxiety with offspring MDD, offspring anxiety, and offspring CD. Covariates were examined in bivariate regressions and were retained in the final models if they were associated with offspring MDD, anxiety, or CD at p<.10, with one exception. The prenatal maternal alcohol use pattern had a large number of missing values due to a low follow-up rate at the 2nd trimester assessment. While it was significantly associated with offspring MDD in the bivariate analyses, it was not significant (p=.638) in the multivariable model and was dropped from the final multivariate model.
Multinomial logistic regression models were created to examine offspring depression, anxiety, and conduct symptoms. None of the symptom levels were normally or Poisson distributed: depression and conduct symptoms were strongly right skewed, with anxiety symptoms also positively skewed although not as strongly. In an effort to balance sample size considerations with maintaining the nuance of symptom scores, each outcome was categorized by its own distribution. For depression, offspring were categorized as having low (0 symptoms, 67.2%), medium (1 or 2 symptoms, 16.8%), or high symptoms (3+ symptoms, 14.9%). Conduct symptoms were similarly categorized as low (0 symptoms, 47.7%), medium (1 or 2 symptoms, 36.2%), or high symptoms (3+ symptoms, 9.4%). Anxiety symptoms had a broader distribution and were categorized into approximate quartiles.
Finally, interaction effects between pre- and postnatal depression and anxiety exposure and offspring sex were examined for each model, as evidence suggests that the effects of exposure to pre- and postnatal depression and anxiety may differ by sex (O'Connor, Heron, & Glover, 2002; O'Connor, Heron, Golding, et al., 2002; O'Connor, et al., 2003). Interaction effects were tested in models independent of additional covariates and were retained in the multivariate models if they were significant at the p<.1 level. All regression analyses were completed using SPSS, version 15 ("SPSS for Windows," 2006).
Results
Sample Characteristics
As shown in Table 1, mothers in this sample were on average 22.9 (SD=3.94) years old and were almost equally divided by race (45.4% Caucasian, 54.6% African American). Almost a third (30.8%) the women were first time mothers, 30.5% had had one previous birth, and 38.6% had had two or more previous births. On average, women had completed 11.82 (SD=1.37) years of education at baseline. The average monthly family income over the study period was $1078 a month, with a great deal of variability (SD=$589). At baseline, 26% of the mothers were employed or attending school. There were a large number of pregnancy (64%), labor (44%), and delivery complications (82%). However, complications included minor events such as minor infections and previous pregnancy losses, so the occurrence of a complication does not itself indicate significant medical morbidity. For the offspring, 47.5% were male and 81.1% lived with their mother as the primary custodian throughout the study period (Table 1).
Table 1.
Descriptive statistics for the total sample (n=577) and by offspring Major Depression, Anxiety, and Conduct Disorder status.
| Covariate | Total Sample Mean (SD) or % |
Offspring Major Depression |
Offspring Anxiety Disorder |
Offspring Conduct Disorder |
||||
|---|---|---|---|---|---|---|---|---|
| No n=499 |
Yes n=78 |
No n=517 |
Yes n=60 |
No n=505 |
Yes n=67 |
|||
| Baseline Maternal Age (yrs) | 22.93 (3.94) | 23.06 (3.96)* | 22.13 (3.78)* | 23.0 (3.98) | 22.37 (3.65) | 22.96 (3.96) | 22.90 (3.84) | |
| Baseline Maternal Race | Caucasian | 45.4 | 43.7** | 56.4** | 44.9 | 50.0 | 45.7 | 43.3 |
| African American | 54.6 | 56.3** | 43.6** | 55.1 | 50.0 | 54.3 | 56.7 | |
| Baseline Prior Births | None | 30.8 | 30.9 | 30.8 | 30.2 | 36.7 | 30.3 | 34.3 |
| 1 | 30.5 | 30.3 | 32.1 | 30.6 | 30.0 | 31.3 | 25.4 | |
| 2+ | 38.6 | 38.9 | 37.2 | 39.3 | 33.3 | 38.4 | 40.3 | |
| Baseline Education (yrs) | 11.82 (1.37) | 11.87 (1.36)** | 11.5 (1.44)** | 11.87 (1.39)** | 11.42 (1.17)** | 11.85 (1.39) | 11.61 (12.7) | |
| Baseline Monthly Family Income | $346 ($293) | $351 ($292) | $312 ($296) | $351 ($293) | $304 ($290) | $350 ($293) | $322 ($279) | |
| Avg. Monthly Family Income throughout study | Low: 0 – $640 | 33.6 | 32.9 | 38.2 | 31.8** | 50.0** | 32.5 | 41.5 |
| Medium: $641 – 1207 | 32.9 | 33.5 | 28.9 | 33.1 | 31.0 | 33.7 | 26.2 | |
| High: $1208+ | 33.4 | 33.5 | 32.9 | 35.1** | 19.0** | 33.7 | 32.3 | |
| Baseline Maternal Employment | Employed or in School | 26 | 27.1* | 19.2* | 73.9 | 75.0 | 26.7 | 19.4 |
| Unemployed | 74 | 72.9* | 80.8* | 26.1 | 25.0 | 73.3 | 80.6 | |
| Pregnancy Complications | No | 36.4 | 36.1 | 38.5 | 37.5* | 26.7* | 36.6 | 35.8 |
| Yes | 63.6 | 63.9 | 61.5 | 62.5* | 73.3* | 63.4 | 64.2 | |
| Labor Complications | No | 56 | 55.6 | 59 | 56.7 | 50.8 | 55.8 | 59.7 |
| Yes | 43.8 | 44.4 | 41 | 43.3 | 49.2 | 44.2 | 40.3 | |
| Delivery Complications | No | 18.7 | 18.6 | 19.2 | 19.3 | 13.3 | 17.4** | 26.9** |
| Yes | 81.3 | 81.4 | 80.8 | 80.7 | 86.7 | 82.6** | 73.1** | |
| Offspring Gender | Male | 47.5 | 51.3**** | 23.1**** | 50.9**** | 18.3**** | 45.7** | 59.7** |
| Female | 52.5 | 48.7**** | 76.9**** | 49.1**** | 81.7**** | 54.3** | 40.3** | |
| Lives with Mother throughout study | Consistently | 81.1 | 80.6 | 84.6 | 80.9 | 83.3 | 81.4 | 77.6 |
| Inconsistently | 18.9 | 19.4 | 15.4 | 19.1 | 16.7 | 18.6 | 22.4 | |
| Avg. Prenatal Alcohol (avg. drinks/day) | .29 (.84) | .28 (.87) | .34 (.55) | .28 (.87) | .32 (.47) | .27 (.86) | .42 (.61) | |
| Prenatal Alcohol | None | 31.54 | 31.81* | 29.85* | 31.40 | 32.69 | 31.91 | 27.27 |
| 1st Trimester only | 27.59 | 27.95* | 25.37* | 27.44 | 28.85 | 27.90 | 27.27 | |
| 1st and 2nd Trimester | 17.63 | 16.63* | 23.88* | 17.44 | 19.23 | 17.49 | 18.18 | |
| All Trimesters | 23.24 | 23.61* | 20.90* | 23.72 | 19.23 | 22.70 | 27.27 | |
| Avg. Postnatal Alcohol (drinks/day) | .74 (1.22) | .68 (1.08)*** | 1.1 (1.91)*** | .73 (1.14) | .78 (1.77) | .71 (1.11) | .96 (1.85) | |
| Avg. Later Alcohol (drinks/day) | .97 (1.47) | .95 (1.49) | .97 (1.47) | .94 (1.48) | 1.23 (1.41) | .91 (1.35) | 1.46 (2.18) | |
| Avg. Prenatal Marijuana (joints/day) | .25 (.67) | .24 (.62) | .32 (.9) | .23 (.6) | .45 (1.8) | .25 (.69) | .22 (.48) | |
| Prenatal Marijuana | None | 56.95 | 56.64 | 59.09 | 56.75 | 58.82 | 56.89 | 57.89 |
| 1st Trimester only | 18.15 | 18.58 | 15.15 | 18.63 | 13.73 | 17.94 | 17.54 | |
| 1st and 2nd Trimester | 6.56 | 6.86 | 4.55 | 6.64 | 5.88 | 5.91 | 12.28 | |
| All Trimesters | 18.34 | 17.92 | 21.21 | 17.99 | 21.57 | 19.26 | 12.28 | |
| Avg. Postnatal Marijuana (joints/day) | .38 (.91) | .37 (.89) | .48 (1.03) | .37 (.88) | .53 (1.12) | .38 (.91) | .43(.91) | |
| Avg. Later Marijuana (joints/day) | .16 (.51) | .16 (.52) | .18 (.48) | .15 (.51) | .25 (.52) | .15 (.48)* | .26 (.72)* | |
| Prenatal Drugs | None | 89.96 | 89.41 | 93.51 | 89.63 | 92.98 | 90.12 | 89.55 |
| 1st Trimester only | 7.92 | 8.55 | 3.90 | 8.22 | 5.26 | 7.46 | 10.45 | |
| 1st and 2nd Trimester | 1.58 | 1.43 | 2.60 | 1.57 | 1.75 | 1.81 | 0.00 | |
| All Trimesters | 0.53 | 0.61 | 0.00 | 0.59 | 0.00 | 0.60 | 0.00 | |
| Postnatal Drugs | None | 80.6 | 80.6 | 80.9 | 81.1 | 76.8 | 80.7 | 79.4 |
| Any | 19.4 | 19.4 | 19.1 | 18.9 | 23.2 | 19.3 | 20.6 | |
| Later Drugs | None | 71.2 | 71.1 | 71.8 | 71.0 | 73.3 | 72.1 | 64.2 |
| Any | 28.8 | 28.9 | 28.2 | 29.0 | 26.7 | 27.9 | 35.8 | |
| Avg. Prenatal Tobacco | <1 per day | 47.3 | 47.9 | 43.6 | 48.4 | 38.3 | 48.1 | 40.3 |
| 1–19 daily | 37.3 | 37.5 | 35.9 | 36.9 | 40.0 | 36.6 | 44.8 | |
| 20+ daily | 15.4 | 14.6 | 20.5 | 14.7 | 21.7 | 15.2 | 14.9 | |
| Avg. Postnatal Tobacco | <1 per day | 38.4 | 39.0 | 33.8 | 39.1 | 32.1 | 39.3 | 28.6 |
| 1–19 daily | 32.0 | 32.4 | 29.4 | 32.4 | 28.6 | 30.8 | 42.9 | |
| 20+ daily | 29.6 | 28.6 | 36.8 | 28.5 | 39.3 | 29.9 | 28.6 | |
| Pre- and Postnatal Depression Trajectory | Low | 16.5 | 16.5 | 17.0 | 16.5 | 17.0 | 17.0 | 12.5 |
| High | 83.5 | 83.5 | 83.0 | 83.5 | 83.0 | 83.0 | 87.5 | |
| Pre- and Postnatal Anxiety Trajectory | Low | 12.0 | 11.4 | 17.0 | 12.0 | 11.3 | 11.6 | 15.6 |
| Medium | 52.6 | 53.2 | 47.2 | 52.6 | 52.8 | 52.5 | 51.6 | |
| High | 35.4 | 35.3 | 35.8 | 35.3 | 35.8 | 35.9 | 32.8 | |
| Average Maternal Depression | 18.98 (9.34) | 18.59*** (9.27) | 21.53*** (9.45) | 18.79 (9.29) | 20.7 (9.65) | 18.73* (9.25) | 21.0* (10.0) | |
| Average Maternal Anxiety | 16.65 (4.61) | 16.45** (4.54) | 18.01** (4.87) | 16.56 (4.58) | 17.46 (4.78) | 16.61 (4.58) | 17.16 (4.89) | |
Note. Prenatal = 1st, 2nd, and 3rd trimesters; postnatal= 8 months and 18 months post-delivery; later=3–16 years post-delivery. Drugs refers to all illicit substances (e.g. heroin, cocaine, etc.) except marijuana, which was measured separately. Statistical comparisons are made within diagnostic categories. Average maternal depression and anxiety is the average of the age 14 and 16 year assessments.
p<.1.
p<.05.
p<.01.
p<.001.
The MHPCD study’s primary objective was to evaluate the effects of maternal prenatal substance use on child development, and therefore, the rates of substance use among the mothers are higher than in the general population. The average alcohol use during pregnancy was .29 drinks per day (SD=.84). However, about 27% of women in this sample did not use any alcohol or marijuana during the prenatal period and 22.2% of women did not use alcohol or other illicit drugs during the prenatal period. Thirty-two percent of women used no alcohol during the prenatal period, 27.6% used only during the first trimester. Average postnatal alcohol use was .74 (SD=1.22) drinks per day, and alcohol use during later assessments (3 to 16 years) averaged .97 (SD=1.47) drinks per day.
Average daily marijuana use was .25 joints per day (SD=.67) during the prenatal period. Almost 57% of women used no marijuana during the prenatal period. Marijuana use in the postnatal period averaged .38 (SD=.91) joints per day, and marijuana use over later assessments (3 to 16 years) averaged .16 (SD=.51) joints per day (Table 1).
Approximately 90% of the women in this sample did not use any illicit drugs (excluding marijuana, which was measured separately) during the prenatal period. Almost 8% reported using illicit drugs in the first trimester only, 1.6% used into the second trimester, and 0.5% used illicit drugs throughout the prenatal period. Nineteen percent of women reported using other illicit drugs during the postnatal period, and 28.8% reported using illicit drugs at one of the later postpartum assessments.
Approximately 47.3% of women averaged less than 1 cigarette a day or abstained during the prenatal period, 37.3% averaged from 1–19 cigarettes per day, and 15.4% averaged 20+ cigarettes per day. In the postnatal period, 38.4% of women averaged less than 1 cigarette per day, 32% averaged between 1 and 19 cigarettes per day, and 29.6% averaged 20 or more cigarettes a day (Table 1).
Maternal depression scores were high. The mean CES-D score was 21.1 (SD=8.7), well above the 16-point cut-off suggested to identify cases of probable depression (Radloff, 1977). The average maternal trait anxiety score was 17.8 (SD=4.8). In the later two waves at offspring ages 14 and 16, maternal CES-D and trait anxiety scores were slightly lower, the mean CES-D score was 18.98 and trait anxiety score was 16.65. Psychiatric illnesses were diagnosed in 32% of offspring at 16 years postnatally. The most common diagnoses were MDD (14%) and CD (12%), followed by PTSD (6%). All other disorders had a prevalence of 5% or less. The three combined anxiety disorders (PTSD, GAD, and Separation Anxiety) had a prevalence of 10%.
Part One: Maternal Symptom Trajectories
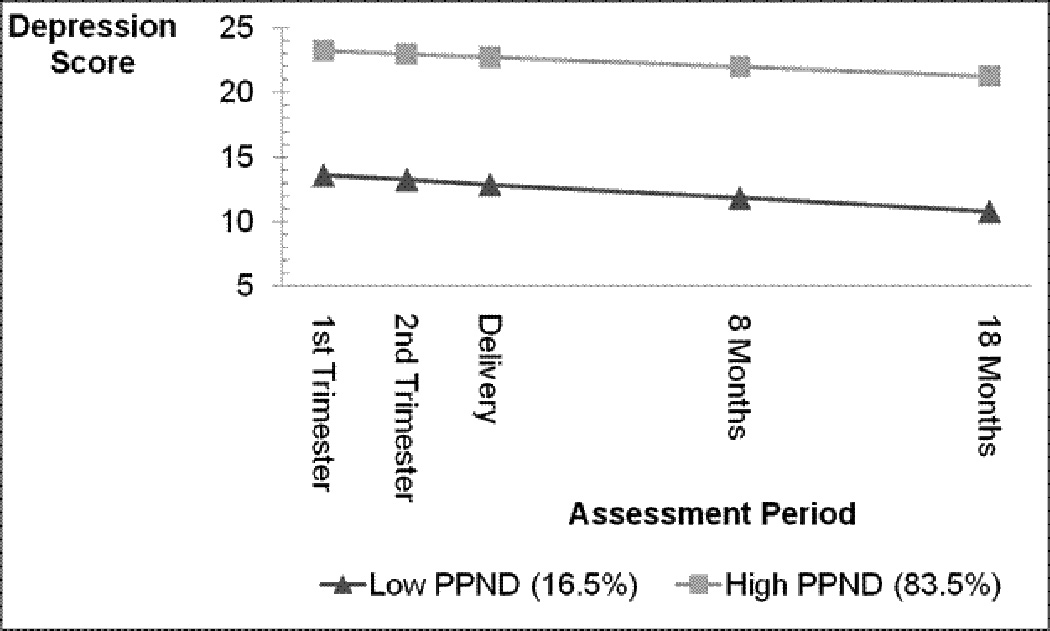
Pre- and postnatal depression. Models using 2 to 5 latent classes of pre-and postnatal depression symptoms were tested to identify homogenous subgroups of depressive symptom patterns across the five assessments (1st, 2nd, 3rd trimesters, and 8 and 18 months postnatally). Models specifying five latent classes all failed to converge. Parameter estimates of linear through cubic growth were tested for each model. There was no evidence of cubic or quadratic growth. All growth-factor and class-specific variances and covariances were freely estimated. The best fit model was a linear two-group solution (Figure 1). The solution suggests a low and a high symptom group that both have a small decrease in symptoms over the five assessment points. Only a small portion (17%) of the sample was classified into the low symptom group, most (84%) were in the high symptom group. The classification means (an indication of how definitively individuals are classified into one group over the other) were 0.69 for the low symptom group and 0.81 for the high symptom group [.7 is considered adequate (White, Nagin, Replogle, & Stouthamer-Loeber, 2004)]. The mean baseline CES-D depression score in the high depression symptom trajectory was 22.9 (SD=.37), whereas the mean CES-D score in the low trajectory group was 11.4 (SD=.47), suggesting that mothers in the high pre- and postnatal depression group had mood scores in a clinically significant range (Radloff, 1977).
Figure 1.
Simple logistic regression was used to evaluate univariate associations among the potential correlates of maternal pre- and postnatal depression symptom trajectory membership. Covariates tested included maternal age, race, number of prior births, baseline marital status, baseline education, baseline monthly family income, baseline maternal employment, pregnancy, labor and delivery complications, baseline social support, pre-pregnancy alcohol, marijuana, cocaine and other illicit drug use, and baseline cigarette use. Of these, education, monthly family income, social support, and cigarette smoking were significantly associated with the depression trajectories (p<.1) and were included in the multivariate model. Table 2 presents the multivariate logistic regression model results for pre- and postnatal depression trajectory group membership. Model diagnostics suggested that all testable assumptions for logistic regression were met.
Table 2.
Multivariate logistic regression results for explanatory variables of depression symptom trajectory membership.
| Variable | β | SD | Wald χ2 | OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Constant | 3.249 | 1.092 | 8.847 | 25.768 | ---- | .003 | |
| Baseline Maternal Education | −.085 | .090 | .897 | .918 | .769–1.096 | .344 | |
| Baseline Family Income | −.070 | .040 | 3.081 | .933 | .863–1.008 | .079 | |
| Baseline Social Support | Low | Reference | ---- | ---- | ---- | ---- | --- |
| High | −.711 | .288 | 6.077 | .491 | .279–.864 | .014 | |
| Baseline Cigarette Use | <1/ day | Reference | ---- | --- | ---- | ---- | --- |
| 1–19 | .356 | .265 | 1.802 | 1.428 | .849–2.401 | .18 | |
| 20+ | 1.103 | .459 | 5.774 | 3.014 | 1.226–7.413 | .016 | |
Model fit: Model χ2=22.37 (7), p<.001; Nagelkerke pseudo R2 =.069
In the full multivariate model, only social support and cigarette smoking were significantly associated with high pre- and postnatal depression trajectory membership. Mothers with high social support were 51% less likely to be in the high pre- and postnatal depression trajectory than those with low social support (OR=.49, CI=.28–.86, p=.014). Women in the high trajectory were three times more likely to smoke more than a pack per day (OR=3.01, CI=1.23–7.41, p=.016) compared to the low pre- and postnatal depression trajectory.
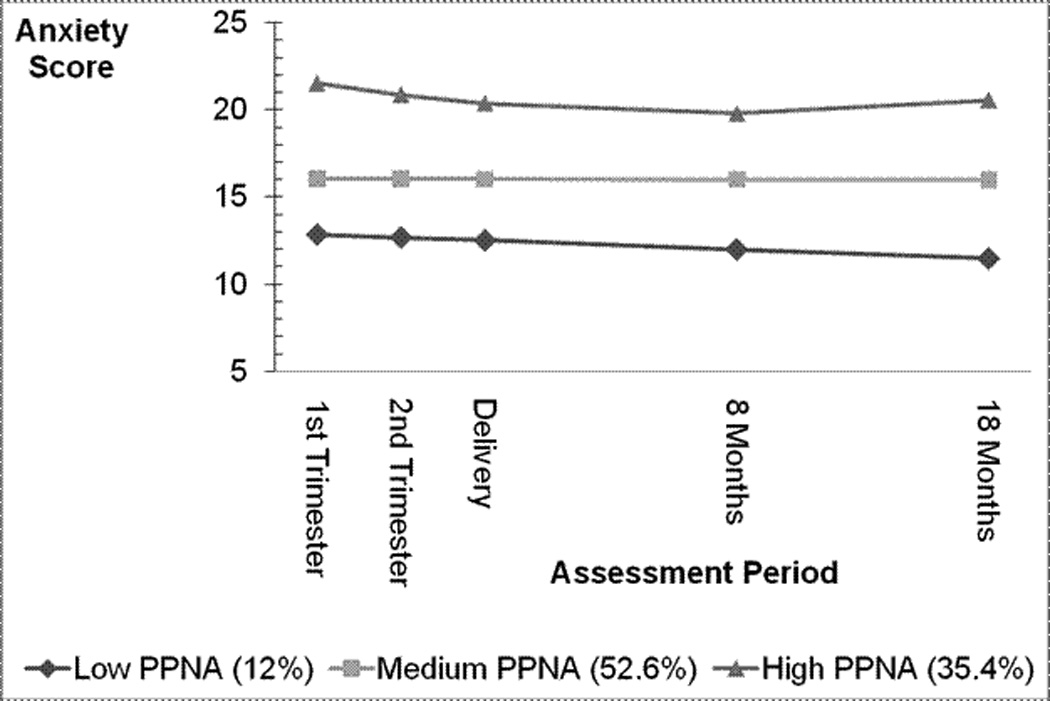
Pre- and postnatal anxiety. Pre- and postnatal anxiety symptom trajectories were modeled in the same fashion as the pre- and postnatal depression symptom trajectories. The best fitting pre- and postnatal anxiety model was a three group trajectory model (low, medium, and high symptoms) with a quadratic growth pattern in the high symptom group (Figure 2). Twelve percent of the sample was classified into the low pre- and postnatal anxiety trajectory, 53% of the sample was classified in the medium pre- and postnatal anxiety group, and 35% of women were in the high pre- and postnatal anxiety group. Symptom levels for all three groups changed little over time. Mothers classified as being in the low pre- and postnatal anxiety trajectory had a mean baseline trait anxiety score of 12.7 (SD=.24), the medium pre- and postnatal anxiety trajectory had a mean baseline anxiety score of 15.8 (SD= .14), and the high pre- and postnatal anxiety group had a mean baseline anxiety score of 22.4 (SD= .3). The classification means were 0.7 for the low group, 0.79 for the medium group, and 0.87 for the high symptom group.
Figure 2.
Individual ordinal regression models with a logit link were used to evaluate univariate associations among the potential correlates of pre- and postnatal anxiety symptom trajectory membership. The correlates were the same as those tested for inclusion in the pre- and postnatal depression trajectories models. For the pre- and postnatal anxiety models, maternal education, monthly family income, social support, and cigarette use were significantly associated with pre- and postnatal anxiety in the bivariate regressions (p<.1) and were included in the multivariate model.
Table 3 presents the results of the ordinal regression model. In the final model, monthly family income and maternal social support were associated with pre- and postnatal anxiety trajectory group membership. For each dollar increase in monthly family income, there was a .06 decrease in the expected log odds of moving into the next higher category of pre- and postnatal anxiety. Compared to mothers with high social support, mothers with low social support had an increase in the expected ordered log odds of 1.0 when moving into the next higher category of pre- and postnatal anxiety. Diagnostic tests indicated that the assumption of parallel lines for ordinal regression was met.
Table 3.
Multivariate ordinal regression results for explanatory variables of anxiety symptom trajectory membership.
| Variable | Estimate | SD | Wald χ2 | 95% CI | p-value | |
|---|---|---|---|---|---|---|
| Baseline Maternal Education | −.087 | .066 | 1.735 | −.216–.042 | .188 | |
| Baseline Family Income | −.059 | .030 | 3.911 | −.118– −.001 | .048 | |
| Baseline Maternal Social Support | Low | Reference | ---- | ---- | ---- | ---- |
| High | −1.002 | .185 | 29.168 | −1.365 – −.638 | <.001 | |
| Baseline Maternal Cigarette Use | <1/ day | Reference | ---- | ---- | ---- | ---- |
| 1–19 | .020 | .190 | .011 | −3.52 –.392 | .916 | |
| 20+ | .454 | .256 | 3.141 | −.048 – .956 | .076 | |
Model Fit: Pearson χ2= 413.662 (393), p=.227; Test of parallel lines: χ2= 4.379 (5), p=.496
Pre- and postnatal depression and anxiety co-occurrence
Two-way chi-square analysis indicated that there was a strong positive association between experiencing pre- and postnatal depression and pre- and postnatal anxiety symptoms (Fisher’s exact=124.67, p<.001). Of the participants who experienced low depression, the majority experienced low or medium anxiety (47.0% and 48.8%, respectively). Only 4.2% of the participants who experienced low depression experienced high anxiety. However, of the participants who experienced high depression, 52.3% and 42.5% experienced medium or high anxiety, respectively. Only 5.1% of the participants who experienced high depression experienced low anxiety.
Part Two: Offspring Mental Health Outcomes
Major depressive disorder and symptoms. Logistic regression was used to determine if offspring MDD by age 16 was predicted by the maternal symptom trajectory. Separate models were tested for pre- and postnatal depression and pre- and postnatal anxiety. Tests of logistic regression assumptions indicated that all assumptions were met except for a small cluster of outliers in the residuals. This was present in each of the three models. There was no evidence of error in the data for these individuals and therefore, they were retained in the final multivariate model. A sensitivity analysis was also performed removing these cases from the data set. The results did not change substantially.
Following the same procedure outlined for regression modeling of the maternal symptom trajectories, covariates were tested using bivariate logistic regression models. Covariates (as shown in Table 1) associated with offspring MDD at p<.1 were included in the full multivariate model. Interactions between sex and pre- and postnatal depression or pre- and postnatal anxiety trajectory membership were tested in each model. Neither interaction was significant. Covariates that were significant (p<.1) and included in the multivariate models were: offspring sex, maternal age, maternal race, maternal education, baseline employment, prenatal alcohol pattern, average postnatal alcohol, and average maternal depression and anxiety scores at the age 14 and 16 waves.
None of the pre- and postnatal depression or pre- and postnatal anxiety symptom trajectories were significantly associated with offspring MDD (pre- and postnatal depression: Wald χ2=2.228 (1), p=.136; pre- and postnatal anxiety Wald χ 2 =5.355 (2), p=.069 (Tables 4 and 5). Of the covariates included in the multivariate model, only offspring sex, maternal age, postnatal alcohol, and maternal depression at the 14- and 16-year follow-ups remained significant after controlling for the other covariates. Male offspring were 87% less likely to develop MDD than female offspring. For each year increase in maternal age at baseline, the odds of MDD in the offspring decreased by about 14%. For each point increase in average maternal postnatal alcohol consumption, the odds of offspring MDD increased by 23%. Each point increase in average maternal depression score at the 14- and 16-year follow-ups was associated with a 7.3% increase in the odds of offspring developing MDD by age 16. Multinomial logistic regression analyses of symptom levels yielded similar results: Neither pre- and postnatal depression nor anxiety symptom exposure was associated with having elevated DIS-IV depression symptoms at age 16 (results not shown).
Table 4.
Logistic regression of offspring major depression predicted by maternal pre- and postnatal depression trajectory groups.
| Variable | β | SD | Wald χ2 |
OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Pre- and Postnatal Depression Trajectory (Ref=Low) | High | −.679 | .455 | 2.228 | 1.237 | .507 - .208 | .136 |
| Gender (Ref=Female) | Male | −1.895 | .460 | 16.990 | .370 | .150 - .061 | <.001 |
| Maternal Age | −.150 | .054 | 7.591 | .861 | .774 - .958 | .006 | |
| Maternal Race (Ref=African American) | .576 | .345 | 2.782 | 1.779 | .904 – 3.500 | .095 | |
| Maternal Education | −.061 | .143 | .179 | .941 | .941 - .711 | .673 | |
| Baseline Maternal Employment | −.481 | .438 | 1.202 | .618 | .262 – 1.460 | .273 | |
| Postnatal Alcohol | .202 | .102 | 3.959 | 1.224 | 1.003 – 1.494 | .047 | |
| Average Maternal Depression | .071 | .031 | 5.218 | 1.073 | 1.010 – 1.140 | .022 | |
| Average Maternal Anxiety | −.077 | .060 | 1.666 | .926 | .824 – 1.041 | .197 | |
Model Chi-Square = 49.06 (9), p <.001, Nagelkerke pseudo R2=.207
Table 5.
Logistic regression of offspring major depression predicted by maternal pre- and postnatal anxiety trajectory groups.
| Variable | β | SD | Wald χ2 | OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Pre- and Postnatal Anxiety Trajectory | Low (Ref) | 5.355 | .069 | ||||
| Medium | −1.131 | .511 | 4.909 | .323 | .119 – .878 | .027 | |
| High | −1.154 | .550 | 4.404 | .315 | .107 – .927 | .036 | |
| Offspring Gender (Ref=Female) | Male | −1.900 | .461 | 16.956 | .150 | .061 – .370 | <.001 |
| Baseline Maternal Age | −.155 | .055 | 7.898 | .856 | .768 – .954 | .005 | |
| Maternal Race (Ref=African American) | .519 | .348 | 2.215 | 1.680 | .848 – 3.325 | .137 | |
| Baseline Maternal Education | −.069 | .144 | .231 | .933 | .703 – 1.238 | .631 | |
| Baseline Maternal Employment | −.476 | .438 | 1.180 | .621 | .263 – 1.467 | .277 | |
| Postnatal Alcohol | .208 | .102 | 4.163 | 1.231 | 1.008 – 1.503 | .041 | |
| Average Maternal Depression | .064 | .031 | 4.274 | 1.066 | 1.003 – 1.132 | .039 | |
| Average Maternal Anxiety | −.059 | .061 | .920 | .943 | .836 – 1.063 | .338 |
Model Chi-Square =51.824 (10), p <.001, Nagelkerke pseudo R2=.218
Anxiety disorders and symptoms. Logistic regression analyses were conducted to examine the association between maternal pre- and postnatal depression and anxiety trajectories on the group of offspring anxiety disorders (PTSD, separation anxiety, and GAD). Evaluation of the logistic regression assumption indicated a small group of residual outliers. Models conducted with and without these individuals did not significantly differ and there was no evidence of data error so these individuals were retained in the final analyses.
In the bivariate models, baseline maternal education, monthly family income averaged across the follow-up years, pregnancy complications, and child sex were associated with anxiety disorders in the offspring (p<.1, Table 1). There was no significant interaction between child sex and maternal pre- and postnatal depression or anxiety in the offspring anxiety models. Pre- and postnatal depression and anxiety were not significantly associated with offspring anxiety disorders by age 16 (Tables 6 and 7). Of the covariates included in the final model, only child sex remained significantly associated with offspring anxiety (p<.001).
Table 6.
Logistic regression of offspring anxiety disorder predicted by maternal pre- and postnatal depression trajectory groups.
| Variable | β | SD | Wald χ2 |
OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Pre- and Postnatal Depression Trajectory (Ref=Low) | High | −.262 | .403 | .423 | .769 | .349 – 1.695 | .516 |
| Gender (Ref=Female) | Male | −1.403 | .367 | 14.577 | .246 | .12 – .505 | <.001 |
| Baseline Maternal Education | −.126 | .125 | 1.022 | .882 | .691 – 1.126 | .312 | |
| Pregnancy Complications (Ref=No) | .508 | .508 | 2.297 | 1.663 | .861 – 3.209 | .130 | |
| Average Family Income | Low | 4.577 | .101 | ||||
| Medium | −.568 | .359 | 2.508 | .567 | .281–1.144 | .113 | |
| High | −.779 | .402 | 3.768 | .459 | .209 – 1.008 | .052 | |
Model Chi-Square = 29.191 (6), p <.001, Nagelkerke pseudo R2=.112
Table 7.
Logistic regression of offspring anxiety disorder predicted by maternal pre- and postnatal anxiety trajectory groups.
| Variable | β | SD | Wald χ2 | OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Pre- and Postnatal Anxiety Trajectory | Low (Ref) | .289 | .865 | ||||
| Medium | −.190 | .496 | .147 | .827 | .313 – 2.186 | .702 | |
| High | −2.76 | .516 | .285 | .759 | .276 – 2.088 | .593 | |
| Offspring Gender (Ref=Female) | Male | −1.411 | .368 | 14.714 | .244 | .119 - .502 | <.001 |
| Pregnancy Complications (Ref=No) | −.126 | .125 | 1.012 | .882 | .691 – 1.126 | .314 | |
| Baseline Maternal Education | .509 | .336 | 2.296 | 1.663 | .861 – 3.212 | .103 | |
| Average Family Income | Low | 4.569 | .102 | ||||
| Medium | −.565 | .359 | 2.478 | .569 | .282 – 1.148 | .115 | |
| High | −.784 | .404 | 3.774 | .456 | .287 – 1.007 | .052 |
Model Chi-Square =29.067 (7), p <.001, Nagelkerke pseudo R2=.112
The results of the multinomial logistic regression analyses of RCMAS anxiety symptoms were slightly different than the diagnostic analyses. Similar to the diagnostic analyses, anxiety trajectory exposure was not associated with offspring anxiety symptoms at age 16. Unlike the diagnostic analyses, however, there was a marginally significant interaction between sex and depression trajectory exposure (p=.079) in the bivariate analyses, which remained significant (p=.043) after controlling for other significant covariates including post-postnatal maternal anxiety and depression symptoms (results not shown). Depression symptom trajectory exposure was not significantly associated with the odds of having medium or medium-high anxiety symptoms for either gender. However, males in the high depression symptom trajectory exposure group were significantly less likely to have high anxiety symptoms than males in the low depression trajectory group (OR=.11, CI=.01–.93, p=.043). Depression symptom trajectory was not associated with anxiety symptoms in females at age 16.
Conduct disorder and symptoms. The logistic regression analyses for CD were performed in the same manner as for MDD. Tests of logistic regression assumptions indicated a cluster of outlying residuals, similar to the MDD and anxiety models. The sensitivity analysis without these outliers showed no significant differences so they were retained in the final multivariate model.
In the bivariate models, only offspring sex, delivery complications, and later (when the child was ≥3 years) maternal marijuana use were associated with CD in offspring (p<.1, Table 1). There was no significant interaction between pre- and postnatal depression and sex in the CD model. Pre- and postnatal depression was not significantly associated with CD in offspring by age 16 (Table 8). Child sex was significantly associated with CD, with males 1.9 times more likely to have CD by age 16 than females (p<.001).
Table 8.
Logistic regression of offspring conduct disorder predicted by maternal pre- and postnatal depression trajectory groups.
| Variable | β | SD | Wald χ2 | OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Pre- and postnatal depression Trajectory (Ref=Low) | High | .128 | .424 | .091 | 1.136 | .495 – 2.067 | .763 |
| Offspring Gender (Ref=Female) | Male | .626 | .281 | 4.974 | 1.870 | 1.079 – 3.242 | .026 |
| Delivery Complications (Ref=No) | Yes | .626 | .281 | 4.974 | .561 | .301 – 1.044 | .068 |
| Later Maternal Marijuana Use | .425 | .212 | 4.039 | 1.530 | 1.011 – 2.316 | .044 | |
| Average Maternal Depression | .024 | .015 | 2.512 | 1.024 | .994 – 1.055 | .113 | |
Model Chi-Square =144.916 (5), p =.011, Nagelkerke pseudo R2=.054.
Pre- and postnatal anxiety trajectory exposure was significantly associated with the occurrence of CD (Table 9). The bivariate analyses indicated a significant interaction between sex and pre- and postnatal anxiety, however this interaction only approached significance in the multivariate model (χ2(2) = 5.129, p=.077). Because of the small sample size of females with CD, this is likely the result of the analysis being underpowered and the interaction was retained in the multivariate model. Female offspring with mothers in the medium pre- and postnatal anxiety trajectory were 75% less likely to meet criteria for CD by age 16 (OR=.249, p=.013) and female offspring with mothers in the high pre- and postnatal anxiety trajectory were 80% less likely to meet CD criteria (OR=.203, p=.009) than those with mothers in the low trajectory. By contrast, male offspring with mothers in the medium pre- and postnatal anxiety trajectory were almost four times more likely to meet CD criteria by age 16 (OR=3.97, p=.046) and male offspring with mothers in the high pre- and postnatal anxiety trajectory were 5.6 times more likely to meet CD criteria (OR=5.631, p=.045) than those with mothers in the low pre- and postnatal anxiety trajectory groups.
Table 9.
Logistic regression of offspring conduct disorder predicted by maternal pre- and postnatal anxiety trajectory groups.
| Variable | β | SD | Wald χ2 | OR | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|
| Females (Ref=low pre- and postnatal anxiety) | Medium pre- and postnatal anxiety | −1.391 | .561 | 6.161 | .249 | .083 – .746 | .013 |
| High pre- and postnatal anxiety | −1.596 | .610 | 6.854 | .203 | .061 – .669 | .009 | |
| Males (Ref=low pre- and postnatal anxiety) | Medium pre- and postnatal anxiety | 1.728 | .861 | 4.025 | 5.631 | 1.041 – 30.469 | .045 |
| High pre- and postnatal anxiety | 1.985 | .906 | 4.797 | 7.281 | 1.232 – 43.023 | .029 | |
| Delivery Complications | −.489 | .321 | 2.317 | .613 | .327 – 1.151 | .128 | |
| Later Maternal Marijuana Use | .381 | .223 | 2.913 | 1.464 | .945 – 2.268 | .088 | |
| Average Maternal Depression | .028 | .015 | 3.623 | 1.029 | .999 – 1.059 | .057 |
Model Chi-Square =20.385 (6), p =.002, Nagelkerke pseudo R2=.073
Notes: Overall Wald χ2 for sex by maternal pre- and postnatal anxiety interaction =5.129 (2), p=.077
Multinomial logistic regression analyses of DIS-IV conduct symptoms yielded slightly different results. Similar to the diagnostic outcome, maternal depression trajectory was not associated with offspring conduct symptoms at age 16 (results not shown). Bivariate analyses indicated a sex by maternal anxiety trajectory interaction, which remained marginally significant in the multivariable model (p=.06). This may have indicated a lack of statistical power for an interaction effect, however it was also noted that significant differences in the odds of having elevated conduct symptoms associated with anxiety trajectory exposure were only evident when comparing the high conduct symptom group to the no symptom group. The covariates were not significantly different between the medium CD symptoms and the no symptom group, with the exception of average income and post-postnatal maternal depression symptoms. In comparing the high conduct symptom group to the no symptom group, there was a significantly lower odds of having high conduct symptoms among females exposed to the high anxiety symptom trajectory compared to those in the low anxiety trajectory group (OR=.22, CI=.07–.66, p=.007) and marginally lower odds of being in the high conduct symptoms group among females exposed to the medium anxiety trajectory compared to the low anxiety trajectory (OR=.32, CI=.09–1.09, p=.069). While trending in the same direction as the diagnostic analyses, with increased odds of high conduct symptoms in males in the medium and high anxiety symptom trajectory groups, these associations were not statistically significant (p>.1).
Discussion
Part One: Maternal Symptom Trajectories
Pre- and postnatal maternal depressive symptoms were prevalent in this sample. The high levels of depressive symptoms are likely a result of recruiting a primarily low SES sample. In this cohort, monthly family income averaged $346 a month, which was well under the poverty threshold in 1982 and 1983 (U.S. Bureau of the Census, 1984). Studies of pregnant women from urban disadvantaged populations (Buss, 1984; Chung, McCollum, Elo, Lee, & Culhane, 2004; Kurtz Landy, Sword, & Ciliska, 2008; Leech, et al., 2006), as well as studies of African Americans (Buss, 1984; Chung, et al., 2004; Orr, Blazer, & James, 2006; Stewart, Dean, Gregorich, Brawarsky, & Haas, 2007), have all found a high prevalence of elevated depressive symptoms. Our findings are in contrast to a recent study that examined trajectories of depressive symptoms in an urban sample and found that most women were in a consistently non-elevated depressive symptom trajectory (Mora et al., 2009). This inconsistency is likely due to differences between the studies in recruitment source, ethnic composition, and timing of assessments. The high rate of depressive symptoms in this analysis could also be due to the sampling design that included a large number of substance-using women. However, 22% of the sample abstained from both alcohol and drug use at baseline. In addition, trajectory group membership was not associated with substance use. Anxiety symptoms were also common in this study, with the majority of participants falling in the medium or high anxiety trajectories. No previous studies were identified that examined trajectories of anxiety over time in pregnant women, so these findings are unique.
Patterns of symptom trajectories suggest that pre- and postnatal depression and anxiety symptoms are stable across pregnancy and the postnatal period, similar to the findings of many cross-sectional prevalence studies (Luoma, et al., 2001; Najman, Andersen, Bor, O'Callaghan, & Williams, 2000; Perren, von Wyl, Burgin, Simoni, & von Klitzing, 2005). These results support recommendations and existing programs for clinician screening in early pregnancy for pre- and postnatal depression and anxiety (Austin, 2004; Gjerdingen & Yawn, 2007; Gordon, Cardone, Kim, Gordon, & Silver, 2006; Smith et al., 2004). Early identification and intervention may lead to improved maternal outcomes and prevent negative consequences of exposure on the children. Moreover, this suggests that future studies examining the impact and underlying mechanisms of prenatal or postnatal maternal symptoms may have difficulty isolating timing effects as it may be difficult to identify women with symptoms occurring only in one period without very large sample sizes.
Maternal depression and anxiety trajectories were highly correlated. Given the high comorbidity of depression and anxiety disorders, it is not surprising that the majority of women who experienced elevated pre- and postnatal depression symptoms also experienced elevated pre- and postnatal anxiety symptoms. Indeed, there is some suggestion that anxiety and depressive symptoms may be manifestations of the same underlying disorder (Boyer, 2000; Chambers, Power, & Durham, 2004; Heron, et al., 2004; Lee et al., 2007; Stuart, Couser, Schilder, O'Hara, & Gorman, 1998; Sutter-Dallay, Giaconne-Marcesche, Glatigny-Dallay, & Verdoux, 2004; World Health Organization, 2001). Thus, children exposed to maternal depression during early development are also likely to be exposed to elevated maternal anxiety. Unfortunately, sample size considerations prevented a more thorough analysis of the effects of the co-occurrence of these symptoms on offspring outcomes. This remains an area for future research.
Despite the high correlation between the two, the pre- and postnatal depression and pre- and postnatal anxiety models were associated with different factors. Cigarette use was uniquely associated with pre- and postnatal depression. Women in the high pre- and postnatal depression trajectory were three times more likely to smoke a pack or more of cigarettes per day than their low pre- and postnatal depression counterparts, which is consistent with the findings of other studies on depression (Cornelius, Ryan, Day, Goldschmidt, & Willford, 2001; Pritchard, 1994). This is a concern as depressive symptoms may interfere with smoking cessation interventions.
Monthly family income was a significant predictor of pre- and postnatal anxiety trajectory group membership. The risk of being in the medium and high pre- and postnatal anxiety trajectories increased as income decreased. It may be that the stressors associated with poverty and insufficient monetary resources place women at a higher risk for sustained anxiety (Murali & Oyebode, 2004).
Social support was the only covariate associated with both pre- and postnatal depression and pre- and postnatal anxiety in the multivariate models. High maternal social support was strongly protective against high pre- and postnatal depression and anxiety trajectory group membership. This is consistent with research suggesting that social support plays a critical role in the experience of depression and anxiety symptoms in pregnant women (Jareethum et al., 2008; Leigh & Milgrom, 2008; Romans, Walton, Herbison, & Mullen, 1992; Surkan, Peterson, Hughes, & Gottlieb, 2006). Clinicians could focus on helping women to increase their social support networks as a means of improving outcomes for pregnant women with elevated pre- and postnatal depression and pre- and postnatal anxiety (Dennis & Kingston, 2008; Lederman, 1995).
Part Two: Offspring Mental Health Outcomes
Major depressive disorder and symptoms. No association was found between maternal pre- and postnatal depression or pre- and postnatal anxiety and MDD or depression symptoms among offspring. This result is in contrast to some prior studies of pre- and postnatal depression, which suggest that there is an association with depression symptoms and offspring MDD (Allen, et al., 1998; Pawlby, et al., 2009). One explanation for this discrepancy is the use of the CES-D and the trait anxiety scale for assessing maternal symptoms as opposed to a diagnostic measure of depression or anxiety disorders. For example, Pawlby et al. (2008) found that when maternal depression was measured using a diagnostic instrument (the Clinical Interview Schedule), 11 year-old offspring exposed to postnatal maternal depression were at four times the risk of having a psychiatric diagnosis. However, when a symptom-based non-diagnostic scale (Edinburgh Postnatal Depression Scale) was used, there was no association between high depression and child psychopathology. Furthermore, these results may be the result of a ceiling effect in maternal symptoms as the majority of women in this study were classified as being in the high depression trajectory. This is not the first study to find high scores on the CES-D in low socioeconomic or predominantly minority samples (Kurtz Landy, et al., 2008; Orr, et al., 2006; Stewart, et al., 2007). However, the CES-D has a positive predictive value of only 53% for MDD and, as such, is an imperfect diagnostic tool (Thomas, Jones, Scarinci, Mehan, & Brantley, 2001). Additionally, the CES-D (as well as the STPI) is a self-report measure that was repeatedly administered. It may be that the mothers’ current psychological status or the repeated administration of the measure affected the results. The repeated measures were 2 to 10 months apart, however, so this may be less of a concern.
Most previous studies that found significant associations between pre- and postnatal depression exposure and outcomes in offspring examined younger children and examined behavioral and emotional problems, not clinical diagnoses (Beck, 1998; Leech, et al., 2006; O'Connor, Heron, & Glover, 2002). It may be that exposure to pre- and postnatal depression symptoms increases problems in younger children but these problems are either time-limited or do not progress into disorders as the children mature. Alternatively, our sample at age 16 may be too young to detect a significant association, since the peak prevalence of MDD occurs in young adulthood (Kessler et al., 2005). As 67% of offspring reported no MDD symptoms, this is a distinct possibility and analyses at a later follow-up age may result in different findings.
With regard to pre- and postnatal anxiety exposure and offspring development of MDD, our results are inconsistent with the large ALSPAC study, which found a link between anxiety exposure and emotional problems. However, ALSPAC differed in that the outcome was not a diagnostic measure and the children were only 4 and 6.75 years of age (O'Connor, Heron, & Glover, 2002; O'Connor, Heron, Golding, et al., 2002; O'Connor, et al., 2003). Further research is needed using diagnostic measures in adolescents and young adults to verify the null association found in our study.
Anxiety disorders and symptoms. Pre- and postnatal depression and pre- and postnatal anxiety symptoms were not associated with having an anxiety disorder at age 16, although pre-and postnatal depression symptom exposure was associated with anxiety symptoms in males. This association was only evident when comparing males in the high anxiety group to males with low levels of anxiety symptoms, which is surprising given that no association was found in the disorder-level analyses. The lack of association between maternal depression symptom trajectory and the development of anxiety disorders among youth is consistent with the results of Allen and colleagues (1998), who found that combined maternal anxiety and depression symptoms in the postnatal period were associated with MDD and DBD but not with anxiety disorders. However, the lack of literature on maternal pre- and postnatal depression symptoms and subsequent anxiety levels in adolescent offspring prevents placing these findings in context. Identifying an association between maternal depression trajectory and high anxiety symptoms in males but not identifying a similar association when examining anxiety diagnoses may indicate a lack of power in the diagnostic analyses, a statistical artifact in the symptom analyses, or a qualitative difference among those who meet strict clinical guidelines for a disorder versus those who have high symptoms but do not necessarily have a clinical level disorder. Replication in other populations with a larger sample size may be able to elucidate these conflicting findings.
Conduct disorder and symptoms. The finding of no association between pre- and postnatal depression and offspring CD or conduct symptoms is not surprising given past research. Only one study was identified that looked at CD and exposure to maternal depression specifically in the prenatal period (Hay, Pawlby, Waters, Perra, & Sharp, 2010). The findings of this study are the reverse of ours; prenatal depression was associated with CD but prenatal anxiety was not. This may reflect the difficulties in separating the effects of two highly correlated outcomes. This is an area of research that requires more work to confirm or refute our findings.
The significant association between pre- and postnatal anxiety and CD is of particular interest, not only because of the strength of the association but also because of the differential effect in males and females, which was also partially replicated in the conduct symptom analyses in females. High pre- and postnatal anxiety exposure was protective against CD in female offspring, but was a significant risk factor for male offspring. This sex difference is not without precedence. CD is far more common among males and these behaviors are more common among males of every culture and every age (Eme, 2007). Prenatal maternal anxiety may be associated with elevated stress hormones, such as cortisol, that are correlated with higher levels of circulating testosterone crossing the placental barrier (Sarkar, Bergman, Fisk, O'Connor, & Glover, 2007; Sarkar, Bergman, O'Connor, & Glover, 2008). In addition, during the prenatal period, male fetuses begin producing large amounts of androgens, particularly testosterone, which is boosted in the presence of testosterone from the mother. Elevated testosterone levels in utero, driven by elevated maternal anxiety, may affect the developing male hypothalamic pituitary adrenal (HPA) axis such that male offspring are primed to display an aggressive response to stress (Sarkar, et al., 2007; Sarkar, et al., 2008). These findings should be interpreted with caution however, as the symptom analyses in males, while trending in the same direction, were not significant. This may indicate that the findings related to CD diagnosis in males are a statistical artifact, or may reflect the more relaxed criteria inherent in using symptom counts. While all of the offspring who met CD diagnostic criteria (11.7%) were classified as being in the “high” CD symptom category, an additional 4.4% were included in the “high” symptom category who had not been classified as having CD because they did not meet the full diagnostic criteria (i.e., clinically significant impairment was not reported, or the symptoms did not all occur within a 12-month period with at least one occurring in the past 6 months.)
The reduction of CD risk in pre- and postnatal anxiety exposed females may result from the same mechanisms that increase the risk in males. Elevated maternal cortisol promotes an increase in testosterone crossing the placenta which, in females does not trigger additional testosterone production but rather suppresses estrogen production. The reduction in estrogen may alter the developing HPA axis to promote subsequent nonaggressive responses in female offspring (Eme, 2007). These hypotheses are preliminary and more research is needed to verify the specific association between prenatal anxiety, HPA axis development, and aggression in males and females.
Another possible explanation for our findings may involve environmental factors such as maternal-child attachment and parenting behaviors. Past studies have found effects of problematic mother-child bonding that differ by sex. For example, Turner (1991) found that insecure attachment in boys was associated with more aggressive behavior towards peers and insecure attachment in girls was associated with more dependent behaviors towards peers. It is possible that maternal anxiety may impact mother-child bonding or parenting approaches, the consequences of which may manifest differently by child sex.
Study Limitations
One potential limitation of this study is that women were recruited on the basis of their alcohol or marijuana use, which may have biased the associations between pre- and postnatal anxiety and offspring anxiety. However, this is unlikely as substance use was not associated with pre- and postnatal depression or pre- and postnatal anxiety symptom trajectories. Furthermore, average alcohol and marijuana use, and the use of other illicit drugs during pregnancy, were not significantly associated with depressive and anxiety symptom trajectories. This mitigates the concern to some extent, although it is possible that substance use may still impact the relationship between maternal symptoms and child diagnoses. Further replication in other samples is in order.
Additionally, there may be some bias in the adolescents’ recall of lifetime symptoms across development. Recall bias in studies of psychopathology is a legitimate concern, particularly as the recall time lengthens. However, studies of biased self-reporting have found reasonably accurate recall of childhood psychopathology (Brewin, Andrews, & Gotlib, 1993). Moreover, studies of adults have found that most recall bias leads to underreporting of disorders, which, if present, would likely bias our results towards the null (Kruijshaar et al., 2005).
Finally, while this study examined the association between maternal pre- and postnatal depression and anxiety symptoms and child outcome, it is not capable of examining the likely mechanisms of effect. Lack of biological measures including genetic data, information on paternal influence and more detailed information on child rearing environment, and psychological states during the postnatal period (e.g., measures of attachment) all limit the ability to draw substantive conclusions on these processes in these analyses. Therefore, this research serves as a preliminary area of research with substantial area for expansion into whether the effects are due to shared genetic risk, child rearing environment, or an interaction between the two.
Conclusions
To our knowledge, this is the first study to examine trajectories of both pre- and postnatal anxiety and pre- and postnatal depression symptoms at the individual level among low SES women. As this high risk and vulnerable population is understudied, this report makes a significant contribution to our understanding of the challenges faced by these families. It may, however, have limited generalizability to other SES groups.
The study design used in this research provides a number of unique contributions to the literature. The use of individual-level trajectory patterns across both the pre- and postnatal period allows for the evaluation of the effect of commonly occurring patterns of symptoms and provides a more realistic measure of exposure than single time point assessments. To date, very few studies on pre- and postnatal depression have used psychiatric diagnoses in adolescents as an endpoint, and no other study has examined the effect of patterns of pre- and postnatal anxiety on psychiatric illnesses.
The strong association found between pre- and postnatal anxiety exposure and the risk of diagnosis of CD in males is an important finding for future research. However, future replication is in order because of the lack of consistency with the conduct symptom analyses. CD is significantly impairing to individuals, their families, and society. It is associated with poor family and peer relationships, school disruption and truancy, conflicts with authority, and criminal and aggressive behavior. It is also a precursor to antisocial personality disorder (Holmes, Slaughter, & Kashani, 2001; Loeber, Burke, & Pardini, 2009). Current treatment programs for CD have small treatment effects, at best, and the identification of risk factors is essential for reducing the morbidity among adolescents, their families, and society (Dretzke et al., 2005; Woolfenden, Williams, & Peat, 2001). Developing interventions for pregnant women with high levels of anxiety may represent a novel approach to prevent the onset of conduct disorder in their offspring.
Acknowledgements
This work was supported by: NIMH (T32 MH015169), Psychiatric Epidemiology Training Program, Program Director: Gale A. Richardson, Ph.D.; NIDA (R01 DA03874), Epidemiological Study of Prenatal Marijuana Exposure, PI: Nancy L. Day, Ph.D.; NIAAA (R01 AA06666), Alcohol Use During Pregnancy: A Longitudinal Study, PI: Nancy L. Day, Ph.D; and NIAAA (K01 AA00312), Childhood Abuse as a Predictor of Adolescent Alcohol Use, PI: Cynthia A. Larkby. The authors wish to thank Dr. Lidush Goldschmidt and Young Jhon for their patient assistance with this project. This work was conducted in partial fulfillment of requirements for the first author’s doctoral degree.
Footnotes
Disclosure: Dr. Swartz has been a consultant to Navigant and SciMed and has received royalties from UpToDate. The other authors have no potential conflicts of interest to disclose.
References
- Achenbach TM, Edelbrock C. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Burlington. VT: University of Vermont, Department of Psychiatry; 1983. [Google Scholar]
- Allen NB, Lewinsohn PM, Seeley JR. Prenatal and perinatal influences on risk for psychopathology in childhood and adolescence. Developmental Psychopathology. 1998;10(3):513–529. doi: 10.1017/s0954579498001722. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. Washington, D.C.: American Psychiatric Association; 2000. [Google Scholar]
- Austin MP. Antenatal screening and early intervention for “perinatal” distress, depression and anxiety: where to from here? Archives of Women's Mental Health. 2004;7(1):1–6. doi: 10.1007/s00737-003-0034-4. [DOI] [PubMed] [Google Scholar]
- Araya R, Hu X, Heron J, Enoch MA, Evans J, Lewis G, Goldman D. Effects of stressful life events, maternal depression and 5-HTTLPR genotype on emotional symptoms in pre-adolescent children. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150(5):670–682. doi: 10.1002/ajmg.b.30888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MP, Hadzi-Pavlovic D, Leader L, Saint K, Parker G. Maternal trait anxiety, depression and life event stress in pregnancy: relationships with infant temperament. Early Human Development. 2005;81(2):183–190. doi: 10.1016/j.earlhumdev.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Austin MP, Leader LR, Reilly N. Prenatal stress, the hypothalamic-pituitary-adrenal axis, and fetal and infant neurobehaviour. Early Human Development. 2005;81(11):917–926. doi: 10.1016/j.earlhumdev.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Avan B, Richter LM, Ramchandani PG, Norris SA, Stein A. Maternal postnatal depression and children's growth and behaviour during the early years of life: exploring the interaction between physical and mental health. Archives of Disease in Childhood. 2010;95(9):690–695. doi: 10.1136/adc.2009.164848. [DOI] [PubMed] [Google Scholar]
- Barker ED, Copeland W, Maughan B, Jaffee SR, Uher R. Relative impact of maternal depression and associated risk factors on offspring psychopathology. The British Journal of Psychiatry. 2012;200(2):124–129. doi: 10.1192/bjp.bp.111.092346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett B, Schaafsma MF, Guzman AM, Parker GB. Maternal anxiety: a 5-year review of an intervention study. Journal of Child Psychology and Psychiatry. 1991;32(3):423–438. doi: 10.1111/j.1469-7610.1991.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Bauer DJ, Curran PJ. Distributional assumptions of growth mixture models: implications for overextraction of latent trajectory classes. Psychological Methods. 2003;8(3):338–363. doi: 10.1037/1082-989X.8.3.338. [DOI] [PubMed] [Google Scholar]
- Bayer JK, Hiscock H, Ukoumunne OC, Price A, Wake M. Early childhood aetiology of mental health problems: a longitudinal population-based study. Journal of Child Psychology and Psychiatry. 2008 doi: 10.1111/j.1469-7610.2008.01943.x. [DOI] [PubMed] [Google Scholar]
- Beck CT. The effects of postpartum depression on child development: a meta-analysis. Archives of Psychiatric Nursing. 1998;12(1):12–20. doi: 10.1016/s0883-9417(98)80004-6. [DOI] [PubMed] [Google Scholar]
- Beeghly M, Weinberg MK, Olson KL, Kernan H, Riley J, Tronick EZ. Stability and change in level of maternal depressive symptomatology during the first postpartum year. Journal of Affective Disorders. 2002;71(1–3):169–180. doi: 10.1016/s0165-0327(01)00409-8. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. American Journal of Epidemiology. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Boyd JH, Weissman MM, Thompson WD, Myers JK. Screening for depression in a community sample. Understanding the discrepancies between depression symptom and diagnostic scales. Archives of General Psychiatry. 1982;39(10):1195–1200. doi: 10.1001/archpsyc.1982.04290100059010. [DOI] [PubMed] [Google Scholar]
- Boyer P. Do anxiety and depression have a common pathophysiological mechanism? Acta psychiatrica Scandinavica Supplimental. 2000;(406):24–29. [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Gotlib IH. Psychopathology and early experience: a reappraisal of retrospective reports. [Review] Psychological Bulletin. 1993;113(1):82–98. doi: 10.1037/0033-2909.113.1.82. [DOI] [PubMed] [Google Scholar]
- Brown L, Wright J. The relationship between attachment strategies and psychopathology in adolescence. Psychology & Psychotherapy: Theory, Research & Practice. 2003;76(4):351–367. doi: 10.1348/147608303770584728. [DOI] [PubMed] [Google Scholar]
- Buss APR. Temperament: early developing personality traits. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984. [Google Scholar]
- Chambers JA, Power KG, Durham RC. The relationship between trait vulnerability and anxiety and depressive diagnoses at long-term follow-up of Generalized Anxiety Disorder. Journal of Anxiety Disorders. 2004;18(5):587–607. doi: 10.1016/j.janxdis.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Chung EK, McCollum KF, Elo IT, Lee HJ, Culhane JF. Maternal depressive symptoms and infant health practices among low-income women. Pediatrics. 2004;113(6):e523–e529. doi: 10.1542/peds.113.6.e523. [DOI] [PubMed] [Google Scholar]
- Coplan RJ, O'Neil K, Arbeau KA. Maternal anxiety during and after pregnancy and infant temperament at three months of age. Journal of Prenatal and Perinatal Psychology and Health. 2005;19(3):199–215. [Google Scholar]
- Cornelius MD, Ryan CM, Day NL, Goldschmidt L, Willford JA. Prenatal tobacco effects on neuropsychological outcomes among preadolescents. Journal of Developmental & Behavioral Pediatrics. 2001;22(4):217–225. doi: 10.1097/00004703-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Dascalu M, Compton WM, Horton JC, Cottler LB. Validity of DIS-IV in diagnosing depression and other psychiatric disorders among substance users. Drug and Alcohol Dependence. 2003;63:37. [Google Scholar]
- Davis E, Snidman N, Wadhwa P, Glynn L, Schetter C, Sandman C. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6(3):319–331. [Google Scholar]
- Day NL, Robles N. Methodological issues in the measurement of substance use. Annals of the New York Academy of Sciences. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy--a review. Neuroscience & Biobehavioral Reviews. 2005;29(2):295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Kingston D. A systematic review of telephone support for women during pregnancy and the early postpartum period. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2008;37(3):301–314. doi: 10.1111/j.1552-6909.2008.00235.x. [DOI] [PubMed] [Google Scholar]
- Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al. The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children. Health Technology Assessment. 2005;9(50) doi: 10.3310/hta9500. iii, ix–x, 1–233. [DOI] [PubMed] [Google Scholar]
- Eme RF. Sex differences in child-onset, life-course-persistent conduct disorder. A review of biological influences. Clinical Psychology Review. 2007;27(5):607–627. doi: 10.1016/j.cpr.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Thorpe K. Changes in depression during and following pregnancy. ALSPAC Study Team. Study of Pregnancy and Children. Paediatric and Perinatal Epidemiology. 1996;10(3):279–293. doi: 10.1111/j.1365-3016.1996.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Risk factors and life processes associated with the onset of suicidal behaviour during adolescence and early adulthood. Psychological Medicine. 2000;30(1):23–39. doi: 10.1017/s003329179900135x. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Racagni G, Riva MA. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Progress in Neurobiology. 2007;81(4):197–217. doi: 10.1016/j.pneurobio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Gjerdingen DK, Yawn BP. Postpartum Depression Screening: Importance, Methods, Barriers, and Recommendations for Practice. The Journal of the American Board of Family Medicine. 2007;20(3):280–288. doi: 10.3122/jabfm.2007.03.060171. [DOI] [PubMed] [Google Scholar]
- Gordon TEJ, Cardone IA, Kim JJ, Gordon SM, Silver RK. Universal perinatal depression screening in an Academic Medical Center. Obstetrics & Gynecology. 2006;107(2, Part 1):342. doi: 10.1097/01.AOG.0000194080.18261.92. [DOI] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Archives of Women's Mental Health. 2003;6(4):263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Hay DF, Pawlby S, Waters CS, Perra O, Sharp D. Mothers' antenatal depression and their children's antisocial outcomes. Child Development. 2010;81(1):149–165. doi: 10.1111/j.1467-8624.2009.01386.x. [DOI] [PubMed] [Google Scholar]
- Hay DF, Pawlby S, Waters CS, Sharp D. Antepartum and postpartum exposure to maternal depression: Different effects on different adolescent outcomes. Journal of Child Psychology and Psychiatry. 2008;49(10):1079–1088. doi: 10.1111/j.1469-7610.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- Heron J, O'Connor TG, Evans J, Golding J, Glover V. The course of anxiety and depression through pregnancy and the postpartum in a community sample. Journal of Affective Disorders. 2004;80(1):65–73. doi: 10.1016/j.jad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Hyvarinen MA, Okada DM, Oh W. Prenatal and intrapartum high-risk screening. I. Prediction of the high-rish neonate. American Journal of Obstetrics & Gynecology. 1973;117(1):1–9. doi: 10.1016/0002-9378(73)90720-5. [DOI] [PubMed] [Google Scholar]
- Holmes SE, Slaughter JR, Kashani J. Risk factors in childhood that lead to the development of conduct disorder and antisocial personality disorder. Child Psychiatry and Human Development Child. 2001;31(3):183–193. doi: 10.1023/a:1026425304480. [DOI] [PubMed] [Google Scholar]
- Horton JC, Compton WM, Cottler LB. Assessing psychiatric disorders among drug users: Reliability of the revised DIS-IV. Washington, DC: 1998. [Google Scholar]
- Huizink AC, de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(9):1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Jareethum R, Titapant V, Chantra T, Sommai V, Chuenwattana P, Jirawan C. Satisfaction of healthy pregnant women receiving short message service via mobile phone for prenatal support: A randomized controlled trial. Journal of The Medical Association of Thailand. 2008;91(4):458–463. [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Klein DN, Lewinsohn PM, Rohde P, Seeley JR, Olino TM. Psychopathology in the adolescent and young adult offspring of a community sample of mothers and fathers with major depression. Psychological Medicine. 2005;35(03):353–365. doi: 10.1017/s0033291704003587. [DOI] [PubMed] [Google Scholar]
- Kruijshaar ME, Barendregt J, Vos T, de Graaf R, Spijker J, Andrews G. Lifetime prevalence estimates of major depression: An indirect estimation method and a quantification of recall bias. European Journal of Epidemiology. 2005;20(1):103–111. doi: 10.1007/s10654-004-1009-0. [DOI] [PubMed] [Google Scholar]
- Kuo WH, Wilson TE, Holman S, Fuentes-Afflick E, O'Sullivan MJ, Minkoff H. Depressive symptoms in the immediate postpartum period among Hispanic women in three U.S. cities. Journal of Immigrant and Minority Health. 2004;6(4):145–153. doi: 10.1023/B:JOIH.0000045252.10412.fa. [DOI] [PubMed] [Google Scholar]
- Kurtz Landy C, Sword W, Ciliska D. Urban women's socioeconomic status, health service needs and utilization in the four weeks after postpartum hospital discharge: findings of a Canadian cross-sectional survey. BMC Health Services Research. 2008;8:203. doi: 10.1186/1472-6963-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman RP. Treatment strategies for anxiety, stress, and developmental conflict during reproduction. Behavioral Medicine. 1995;21(3):113–122. doi: 10.1080/08964289.1995.9933749. [DOI] [PubMed] [Google Scholar]
- Lee AM, Lam SK, Sze Mun Lau SM, Chong CS, Chui HW, Fong DY. Prevalence, course, and risk factors for antenatal anxiety and depression. Obstetrics & Gynecology. 2007;110(5):1102–1112. doi: 10.1097/01.AOG.0000287065.59491.70. [DOI] [PubMed] [Google Scholar]
- Leech SL, Larkby CA, Day R, Day NL. Predictors and correlates of high levels of depression and anxiety symptoms among children at age 10. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(2):223–230. doi: 10.1097/01.chi.0000184930.18552.4d. [DOI] [PubMed] [Google Scholar]
- Leigh B, Milgrom J. Risk factors for antenatal depression, postnatal depression and parenting stress. BMC Psychiatry. 2008;8:24. doi: 10.1186/1471-244X-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman B, Parmelee AH., Jr Medical correlates of infant development. Pediatrics. 1978;61(3):470–474. doi: 10.1542/peds.61.3.470. [DOI] [PubMed] [Google Scholar]
- Lo YT, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- Loeber R, Burke J, Pardini DA. Perspectives on oppositional defiant disorder, conduct disorder, and psychopathic features. Journal of Child Psychology and Psychiatry. 2009;50(1–2):133–142. doi: 10.1111/j.1469-7610.2008.02011.x. [DOI] [PubMed] [Google Scholar]
- Luoma I, Tamminen T, Kaukonen P, Laippala P, Puura K, Salmelin R, et al. Longitudinal study of maternal depressive symptoms and child well-being. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(12):1367–1374. doi: 10.1097/00004583-200112000-00006. [DOI] [PubMed] [Google Scholar]
- McCartney K, Owen MT, Booth CL, Clarke-Stewart A, Vandell DL. Testing a maternal attachment model of behavior problems in early childhood. Journal of Child Psychology and Psychiatry. 2004;45(4):765–778. doi: 10.1111/j.1469-7610.2004.00270.x. [DOI] [PubMed] [Google Scholar]
- McMahon CA, Barnett B, Kowalenko NM, Tennant CC. Maternal attachment state of mind moderates the impact of postnatal depression on infant attachment. Journal of Child Psychology and Psychiatry. 2006;47(7):660–669. doi: 10.1111/j.1469-7610.2005.01547.x. [DOI] [PubMed] [Google Scholar]
- Mora PA, Bennett IM, Elo IT, Mathew L, Coyne JC, Culhane JF. Distinct trajectories of perinatal depressive symptomatology: evidence from growth mixture modeling. American Journal of Epidemiology. 2009;169(1):24–32. doi: 10.1093/aje/kwn283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali V, Oyebode F. Poverty, social inequality and mental health. Advances in Psychiatric Treatment. 2004;10(3):216–224. [Google Scholar]
- Murray L, Arteche A, Fearon P, Halligan S, Goodyer I, Cooper P. Maternal postnatal depression and the development of depression in offspring up to 16 years of age. Journal of the American Academy of Child & Adolescent Psychiatry. 2011 doi: 10.1016/j.jaac.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Fifth ed. Los Angeles, CA: Muthén & Muthén; 1998–2007. [Google Scholar]
- Najman JM, Andersen MJ, Bor W, O'Callaghan MJ, Williams GM. Postnatal depression-myth and reality: maternal depression before and after the birth of a child. Social Psychiatry and Psychiatric Epidemiology. 2000;35(1):19–27. doi: 10.1007/s001270050004. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Glover V. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(12):1470–1477. doi: 10.1097/00004583-200212000-00019. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children's behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. The British Journal of Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Heron J, Golding J, Glover V. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. Journal of Child Psychology and Psychiatry. 2003;44(7):1025–1036. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- Orr ST, Blazer DG, James SA. Racial disparities in elevated prenatal depressive symptoms among black and white women in eastern north Carolina. Annals of Epidemiology. 2006;16(6):463–468. doi: 10.1016/j.annepidem.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J, Chambers W, Tabrizi MA, Johnson R. Retrospective assessment of prepubertal major depression with the Kiddie-SADS-e. Journal of the American Academy of Child and Adolescent Psychiatry. 1982;21(4):392–397. doi: 10.1016/s0002-7138(09)60944-4. [DOI] [PubMed] [Google Scholar]
- Papousek M, von Hofacker N. Persistent crying in early infancy: a non-trivial condition of risk for the developing mother-infant relationship. Child: Care, Health and Development. 1998;24(5):395–424. doi: 10.1046/j.1365-2214.2002.00091.x. [DOI] [PubMed] [Google Scholar]
- Pastor DL. The quality of mother-infant attachment and its relationship to toddlers' initial sociability with peers. Developmental Psychology. 1981;17(3):326–335. [Google Scholar]
- Pawlby S, Hay DF, Sharp D, Waters CS, O'Keane V. Antenatal depression predicts depression in adolescent offspring: prospective longitudinal community-based study. Journal of Affective Disorders. 2009;113(3):236–243. doi: 10.1016/j.jad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- Pawlby S, Sharp D, Hay D, O'Keane V. Postnatal depression and child outcome at 11 years: The importance of accurate diagnosis. Journal of Affective Disorders. 2008;107(1):241–245. doi: 10.1016/j.jad.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Pemberton CK, Neiderhiser JM, Leve LD, Natsuaki MN, Shaw DS, Reiss D, Ge X. Influence of parental depressive symptoms on adopted toddler behaviors: An emerging developmental cascade of genetic and environmental effects. Devlopment and Psychopathology. 2010;22(04):803–818. doi: 10.1017/S0954579410000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perren S, von Wyl A, Burgin D, Simoni H, von Klitzing K. Depressive symptoms and psychosocial stress across the transition to parenthood: associations with parental psychopathology and child difficulty. Journal of Psychosomatic Obstetrics & Gynecology. 2005;26(3):173–183. doi: 10.1080/01674820400028407. [DOI] [PubMed] [Google Scholar]
- Pritchard CW. Depression and smoking in pregnancy in Scotland. Journal of Epidemiology and Community Health. 1994;48(4):377–382. doi: 10.1136/jech.48.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ree MJ. Distinguishing cognitive and somatic dimensions of state and trait anxiety: Development and validation of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA) Behavioural and Cognitive Psychotherapy. 2008;36(3):313–332. [Google Scholar]
- Reynolds C, Richmond B. What I think and feel: A revised measure of children's manifest anxiety. Journal of Abnormal Child Psychology. 1978;6(2):271–280. doi: 10.1007/BF00919131. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) 2000 [Google Scholar]
- Robles N, Day NL. Recall of alcohol consumption during pregnancy. Journal of Studies on Alcohol and Drugs. 1990;51(5):403–407. doi: 10.15288/jsa.1990.51.403. [DOI] [PubMed] [Google Scholar]
- Romans SE, Walton VA, Herbison GP, Mullen PE. Social networks and psychiatric morbidity in New Zealand women. Australian and New Zealand Journal of Psychiatry. 1992;26(3):485–492. doi: 10.3109/00048679209072075. [DOI] [PubMed] [Google Scholar]
- Sarkar P, Bergman K, Fisk NM, O'Connor TG, Glover V. Amniotic fluid testosterone: relationship with cortisol and gestational age. Clinical Endocrinology. 2007;67(5):743–747. doi: 10.1111/j.1365-2265.2007.02955.x. [DOI] [PubMed] [Google Scholar]
- Sarkar P, Bergman K, O'Connor TG, Glover V. Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: possible implications for foetal programming. Journal of Neuroendocrinology. 2008;20(4):489–496. doi: 10.1111/j.1365-2826.2008.01659.x. [DOI] [PubMed] [Google Scholar]
- Schindler A, Thomasius R, Sack PM, Gemeinhardt B, Kustner U. Insecure family bases and adolescent drug abuse: a new approach to family patterns of attachment. Attachment & Human Development. 2007;9(2):111–126. doi: 10.1080/14616730701349689. [DOI] [PubMed] [Google Scholar]
- Smith MV, Rosenheck RA, Cavaleri MA, Howell HB, Poschman K, Yonkers KA. Screening for and detection of depression, panic disorder, and PTSD in public-sector obstetric clinics. Psychiatric Services. 2004;55(4):407–414. doi: 10.1176/appi.ps.55.4.407. [DOI] [PubMed] [Google Scholar]
- Speilberger CD. Preliminary manual for the State-Trait Personality Inventory (STPI) Tampa, FL: University of South Florida; 1979. [Google Scholar]
- Speilberger CD, Gorsuch R, Lushene R, Vagg P, Jacobs G. Manual for the State-Trait Anxiety Inventory: STAI. San Diego, CA: Mindgarden; 1983. [Google Scholar]
- SPSS for Windows (Version 15) Chicago: SPSS Inc.; 2006. [Google Scholar]
- Stewart AL, Dean ML, Gregorich SE, Brawarsky P, Haas JS. Race/ethnicity, socioeconomic status and the health of pregnant women. Journal of Health Psychology. 2007;12(2):285–300. doi: 10.1177/1359105307074259. [DOI] [PubMed] [Google Scholar]
- Stuart S, Couser G, Schilder K, O'Hara MW, Gorman L. Postpartum anxiety and depression: onset and comorbidity in a community sample. The Journal of Nervous and Mental Disease. 1998;186(7):420–424. doi: 10.1097/00005053-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Peterson KE, Hughes MD, Gottlieb BR. The role of social networks and support in postpartum women's depression: a multiethnic urban sample. Matern Child Health Journal. 2006;10(4):375–383. doi: 10.1007/s10995-005-0056-9. [DOI] [PubMed] [Google Scholar]
- Sutter-Dallay AL, Giaconne-Marcesche V, Glatigny-Dallay E, Verdoux H. Women with anxiety disorders during pregnancy are at increased risk of intense postnatal depressive symptoms: a prospective survey of the MATQUID cohort. European Psychiatry. 2004;19(8):459–463. doi: 10.1016/j.eurpsy.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Sutter-Dallay AL, Murray L, Dequae-Merchadou L, Glatigny-Dallay E, Bourgeois ML, Verdoux H. A prospective longitudinal study of the impact of early postnatal vs. chronic maternal depressive symptoms on child development. European Psychiatry. 2010 doi: 10.1016/j.eurpsy.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Jones GN, Scarinci IC, Mehan DJ, Brantley PJ. The utility of the CES-D as a depression screening measure among low-income women attending primary care clinics. The Center for Epidemiologic Studies-Depression. International Journal of Psychiatry in Medicine. 2001;31(1):25–40. doi: 10.2190/FUFR-PK9F-6U10-JXRK. [DOI] [PubMed] [Google Scholar]
- Turner PJ. Relations between Attachment, Gender, and Behavior with Peers in Preschool. Child Development. 1991;62(6):1475–1488. [PubMed] [Google Scholar]
- U.S. Bureau of the Census. Money, income, and poverty status of families and persons in the United States: 1983 (Advance Data from the March 1984 Current Population Survey. 1984 Retrieved from http://www2.census.gov/prod2/popscan/p60-145.pdf. [PubMed]
- Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Development. 2004;75(4):1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Verbeek T, Bockting CLH, van Pampus MG, Ormel J, Meijer JL, Hartman CA, Burger H. Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. Journal of Affective Disorders. 2011 doi: 10.1016/j.jad.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Ward A, Ramsay R, Turnbull S, Benedettini M, Treasure J. Attachment patterns in eating disorders: past in the present. International Journal of Eating Disorders. 2000;28(4):370–376. doi: 10.1002/1098-108x(200012)28:4<370::aid-eat4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Ward A, Ramsay R, Turnbull S, Steele M, Steele H, Treasure J. Attachment in anorexia nervosa: a transgenerational perspective. British Journal of Medical Psychology. 2001;74(Pt 4):497–505. doi: 10.1348/000711201161145. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Gammon GD, John K, Merikangas KR, Warner V, Prusoff BA, Sholomskas D. Children of Depressed Parents: Increased Psychopathology and Early Onset of Major Depression. Archives of General Psychiatry. 1987;44(10):847–853. doi: 10.1001/archpsyc.1987.01800220009002. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Leckman JF, Merikangas KR, Gammon GD, Prusoff BA. Depression and anxiety disorders in parents and children: Results from the Yale Family Study. Archives of General Psychiatry. 1984;41(9):845. doi: 10.1001/archpsyc.1984.01790200027004. [DOI] [PubMed] [Google Scholar]
- West M, George C. Attachment and dysthymia: the contributions of preoccupied attachment and agency of self to depression in women. Attachment & Human Development. 2002;4(3):278–293. doi: 10.1080/14616730210167258. [DOI] [PubMed] [Google Scholar]
- Westen D, Nakash O, Thomas C, Bradley R. Clinical assessment of attachment patterns and personality disorder in adolescents and adults. Journal of Consulting & Clinical Psychology. 2006;74(6):1065–1085. doi: 10.1037/0022-006X.74.6.1065. [DOI] [PubMed] [Google Scholar]
- White HR, Nagin D, Replogle E, Stouthamer-Loeber M. Racial differences in trajectories of cigarette use. Drug and Alcohol Dependence. 2004;76(3):219–227. doi: 10.1016/j.drugalcdep.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Woolfenden SR, Williams K, Peat J. Family and parenting interventions in children and adolescents with conduct disorder and delinquency aged 10–17. Cochrane Database of Systematic Reviews. 2001;(2):CD003015. doi: 10.1002/14651858.CD003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. World health report 2001. Mental health: new understanding, new hope. Geneva: World Health Organization; 2001. [Google Scholar]
- Yoo HI, Kim BN, Shin MS, Cho SC, Hong KE. Parental attachment and its impact on the development of psychiatric manifestations in school-aged children. Psychopathology. 2006;39(4):165–174. doi: 10.1159/000092677. [DOI] [PubMed] [Google Scholar]
- Zax M, Sameroff AJ, Babigian HM. Birth outcomes in the offspring of mentally disordered women. American Journal of Orthopsychiatry. 1977;47(2):218–230. doi: 10.1111/j.1939-0025.1977.tb00977.x. [DOI] [PubMed] [Google Scholar]


