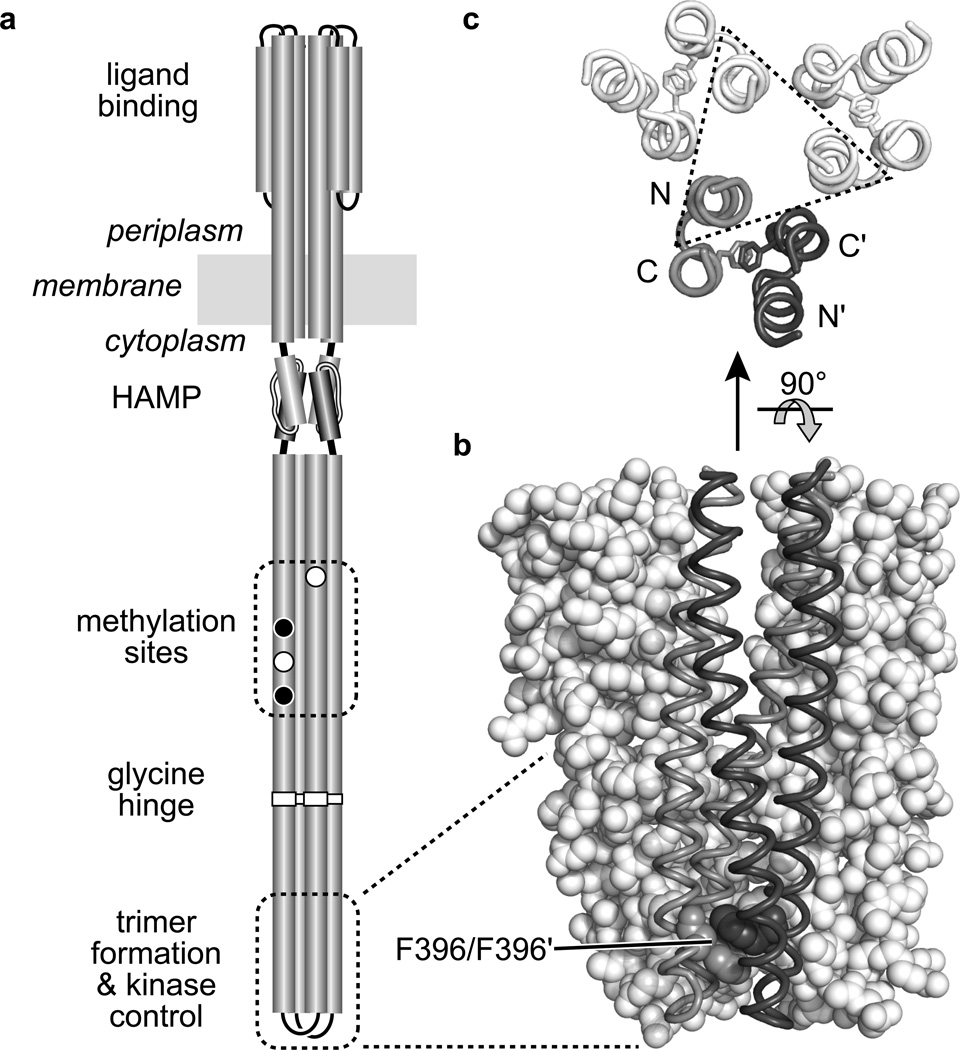
Fig. 1. Structure-function features of the E. coli serine chemoreceptor Tsr.
a. Cartoon of the Tsr homodimer. Cylindrical segments represent α-helical regions, drawn approximately to scale. The entire cytoplasmic portion below the HAMP domain comprises a four-helix, antiparallel, coiled-coil bundle. Methylation sites shown as black circles indicate glutamine (Q) residues; white circles represent glutamate (E) residues. The hairpin tip of the cytoplasmic bundle contains residues that promote interactions with other receptor molecules to form trimers of dimers and with the cytoplasmic CheW and CheA proteins to form ternary signaling complexes.
b. Tsr residues 350–430 in trimer-of-dimers association Two dimers are shown space-filled (white), the third is shown in backbone trace with one subunit shaded gray and the other dark gray. The stacked F396 residues in the third dimer are shown space-filled.
c. Tsr residues 380–400 in trimer-of-dimers association, viewed from the membrane toward the tip. Backbone traces of the four helices in each dimer are shown, with the F396 residues indicated as sticks. Most of the trimer-stabilizing side-chain interactions (not shown) take place between tip residues in one N-terminal helix from each dimer (enclosed in the dashed triangle).