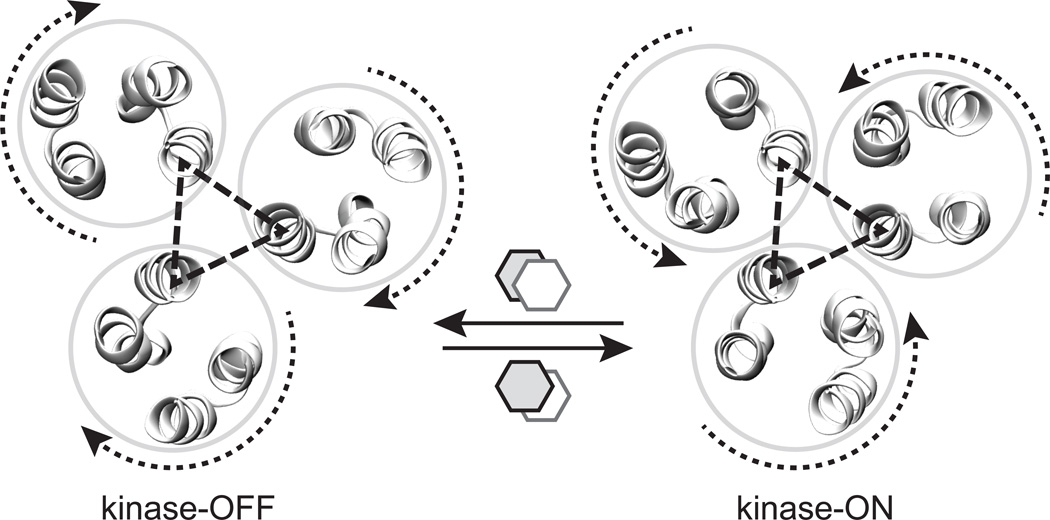
Fig. 5. Model for modulation of kinase activity by receptor trimers of dimers.
Shown are cross-section views of the four-helix bundles near the signaling tip of receptor dimers. The N helices of three receptors, connected by dashed triangles, interact to form trimers of dimers that remain associated throughout the receptors' signaling cycle. The model proposes two signaling conformations at the receptor tip that are stabilized by alternative stacking arrangements of Phe396 residues in each receptor dimer. The signaling states differ by concerted motions of the outer helices in each dimer, which make binding contacts to the CheW coupling protein and the CheA kinase (not shown). To access the kinase-off state, the N and C' helices move further apart and the C and N' helices move closer together. To access the kinase-on state, those motions are reversed. The proposed dynamics of the trimer of dimers is shown in Supplementary Movie 3.