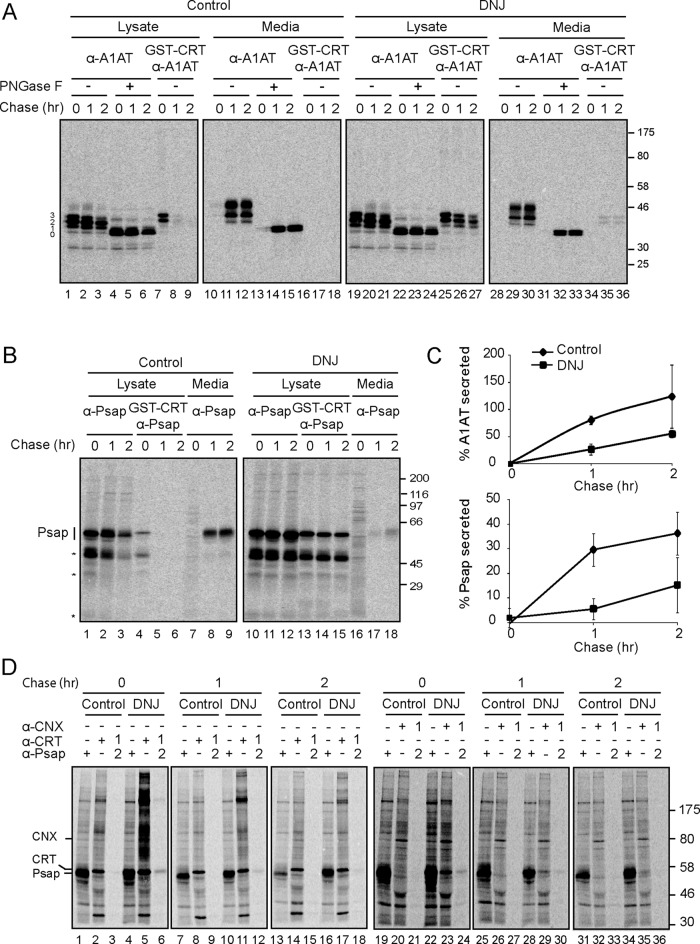
FIGURE 2:
Trapping WT A1AT and the endogenous substrate prosaposin (Psap) in the monoglucosylated state reduced their secretion efficiency and prolonged chaperone binding. (A) MI8-5 Chinese hamster ovary cells were transiently transfected with WT A1AT, radiolabeled for 30 min, and chased for the indicated times. DNJ, 0.5 mM, was added to the pulse and chase media where indicated. WT A1AT was isolated with A1AT antisera from 12% of the cell lysate or the media, and an equal fraction was treated with PNGase F. Monoglucosylated WT A1AT was isolated by GST-CRT pull down, followed by immunoprecipitation with A1AT antisera from 50% of the cell lysate or the media. All samples were resolved on a 9% SDS–PAGE reducing gel. (B) MI8-5 Chinese hamster ovary cells were radiolabeled for 30 min and chased for the indicated times. DNJ, 0.5 mM, was added to the pulse and chase media where indicated. Psap was isolated with Psap antisera from 30% of the lysate or the media. Monoglucosylated Psap was isolated by GST-CRT pull down, followed by immunoprecipitation with Psap antisera from 60% of the cell lysate. All samples were resolved on a 9% SDS–PAGE reducing gel. The asterisk indicates cleaved Psap products. (C) Quantification of the percentage of the fully glycosylated (top band) WT A1AT (top) and endogenous prosaposin (bottom) secreted into the media as a percentage of synthesis. The error bars are representative of the SD from three independent experiments. (D) MI8-5 Chinese hamster ovary cells were radiolabeled for 1 h and chased for the indicated times. Psap was isolated from 5% of the lysate with Psap antisera and calreticulin (CRT)- and calnexin (CNX)-bound species from 15% of the lysate with their respective antisera. For sequential immunoprecipitations, 65% of the lysate was sequentially immunoprecipitated as indicated. All samples were resolved via 7.5% reducing SDS–PAGE.