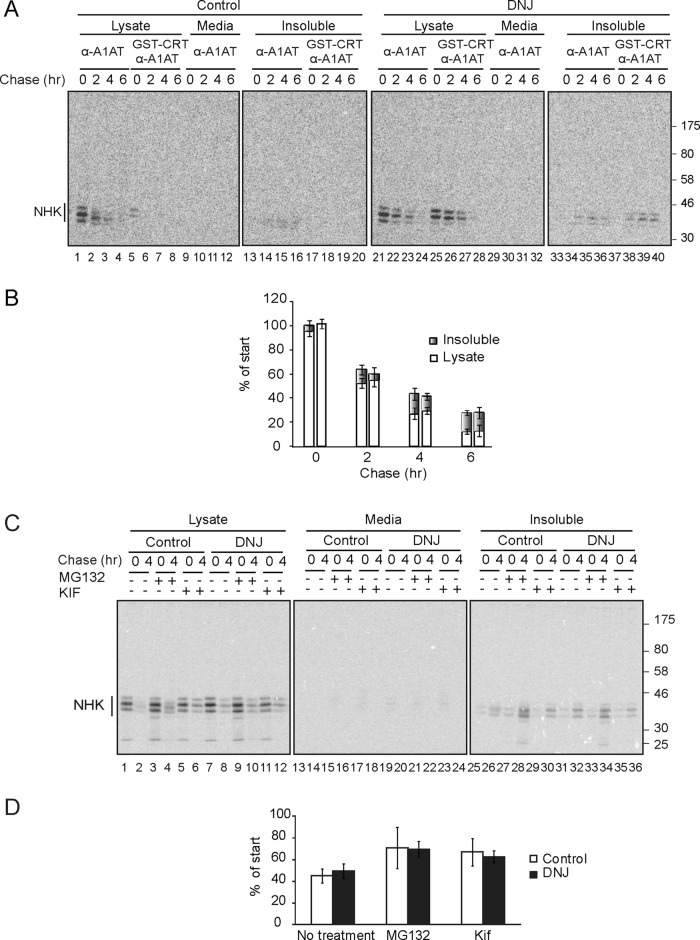
FIGURE 3:
Trapping NHK in the monoglucosylated state did not change its degradation rate. (A) MI8-5 Chinese hamster ovary cells were transiently transfected with NHK, radiolabeled for 30 min, and chased for the indicated times. DNJ, 0.5 mM, was added to the pulse and chase media where indicated. NHK was isolated with A1AT antisera from 10% of the lysate, the medium, or the Triton X-100–insoluble fraction. Monoglucosylated NHK was isolated by GST-CRT pull down, followed by immunoprecipitation with A1AT antisera from 80% of the cell lysate or the Triton X-100–insoluble fraction. All samples were resolved on a 9% SDS–PAGE reducing gel. (B) Quantification of the percentage of NHK from the lysate and the Triton X-100–insoluble fraction as a percentage of synthesis. The first bar for each time point corresponds to control conditions, and the second corresponds to DNJ treatment. All bands were quantified because the fully glycosylated (NHK top band) was not always well resolved in the later time points or the insoluble fraction. The error bars are representative of the SD of three independent experiments. (C) Cells were treated as in A. MG132 (20 μM) or kifunensine (100 μM) was added 4 and 2 h, respectively, before the pulse and included in the pulse and the chase where indicated. All samples were resolved on a 9% reducing SDS–PAGE gel. (D) Quantification of the percentage of the sum of NHK from the cell lysate and the Triton X-100–insoluble fraction as a percentage of synthesis (4-h chase). The error bars are representative of the SD of three independent experiments.