Cells can take up particles by both opsonized and nonopsonized pathways. Silica and latex, but not zymosan, can be taken up by the nonopsonized pathway. Uptake of silica, but not latex, is toxic to macrophages. Nonopsonized phagocytosis is characterized and found to have key differences from the complement- and antibody-opsonized pathways.
Abstract
Silica inhalation leads to the development of the chronic lung disease silicosis. Macrophages are killed by uptake of nonopsonized silica particles, and this is believed to play a critical role in the etiology of silicosis. However, the mechanism of nonopsonized-particle uptake is not well understood. We compared the molecular events associated with nonopsonized- and opsonized-particle phagocytosis. Both Rac and RhoA GTPases are activated upon nonopsonized-particle exposure, whereas opsonized particles activate either Rac or RhoA. All types of particles quickly generate a PI(3,4,5)P3 and F-actin response at the particle attachment site. After formation of a phagosome, the events related to endolysosome-to-phagosome fusion do not significantly differ between the pathways. Inhibitors of tyrosine kinases, actin polymerization, and the phosphatidylinositol cascade prevent opsonized- and nonopsonized-particle uptake similarly. Inhibition of silica particle uptake prevents silica-induced cell death. Microtubule depolymerization abolished uptake of complement-opsonized and nonopsonized particles but not Ab-opsonized particles. Of interest, regrowth of microtubules allowed uptake of new nonopsonized particles but not ones bound to cells in the absence of microtubules. Although complement-mediated uptake requires macrophages to be PMA-primed, untreated cells phagocytose nonopsonized silica and latex. Thus it appears that nonopsonized-particle uptake is accomplished by a pathway with unique characteristics.
INTRODUCTION
Alveolar macrophages play a major role in the immune response to foreign materials and pathogens that enter the body through the lungs (Gordon, 1995). Macrophages have cell surface receptors that have evolved to recognize antibodies or complement factors bound to pathogens or molecular signatures unique to pathogens (e.g., mannose polymers). The molecular mechanisms by which alveolar macrophages initially interact with inhaled environmental particles such as silica, however, are not clear. There is some evidence that scavenger receptors play a role in this process, particularly scavenger receptor-A (SR-A; Kobzik, 1995; Palecanda and Kobzik, 2001; Taylor et al., 2005). However, SR-A receptors depend on negatively charged ligands, and nonopsonized-particle uptake by macrophages occurs regardless of particle charge (Gilberti et al., 2008). Even when SR-A function is compromised by pretreatment with an SR-A inhibitor or use of a knockout mouse model, uptake of silica particles still occurs (Beamer and Holian, 2005; Hamilton et al., 2006; Thakur et al., 2009). These data suggest that SR-A receptors are only partially involved in phagocytosing silica particles.
Regardless of the mechanism of cell–particle interaction, macrophages rapidly internalize silica particles by phagocytosis. Silica particle uptake is lethal to macrophages, and it is this toxicity that is believed to be an important contributor to the development of silicosis, as suggested by in vivo and in vitro studies (Iyer et al., 1996; Iyer and Holian, 1997; Hamilton, 2000; Chao et al., 2001; Thibodeau et al., 2003, 2004; Beamer and Holian, 2005). Within hours of particle exposure, alveolar macrophages have died by apoptosis or necrosis (Gilberti et al., 2008; Joshi and Knecht, 2013). Because latex particles can be taken up as well and are completely nontoxic, there is something about the chemistry of silica particles that is responsible for the surprisingly dramatic effect of such a ubiquitous and seemingly innocuous environmental material. After uptake, phagolysosomal membranes become compromised, leading to leakage of their contents into the cytoplasm (Thibodeau et al., 2004). Many hours later, mitochondrial hyperpolarization, caspase-9 and -3 activation, nuclear changes, and eventually mitochondrial depolarization, phosphatidylserine exposure, and cell death occur in a temporal sequence (Shen et al., 2001; Thibodeau et al., 2003; Costantini et al., 2011; Joshi and Knecht, 2013).
Of the known phagocytosis pathways, Fcγ receptor IIA (FcR)–mediated uptake has been studied in most detail. When an antibody (Ab)-opsonized particle comes in contact with a phagocyte, the Fc domain region of the antibody is bound by transmembrane surface receptors to trigger phagocytosis (Griffin et al., 1976; Greenberg and Grinstein, 2002). Cross-linking of Fc receptors leads to activation by Src family tyrosine kinases Hck, Fgr, and Lyn (Ghazizadeh et al., 1994), which phosphorylate the ITAM regions of the receptor (Daeron, 1997). The tandem SH2 domains of Syk bind to the ITAMs of the receptor, and Syk is activated by autophosphorylation (Kiefer et al., 1998; Fitzer-Attas et al., 2000). Phospholipase D then activates GTPase ARF6 to activate the Rho family small GTPases (Caumont et al., 1998; Honda et al., 1999; Caron, 2000). Cdc42 and Rac1 play a role in actin filament polymerization, which drives membrane around the particle, leading to phagosome formation (Cox et al., 1997; Caron and Hall, 1998). Rho activation also occurs but may not be required for Fc receptor-mediated uptake (Hackam et al., 1997; Caron and Hall, 1998).
Along with GTPase activation, a phosphatidylinositol lipid signaling cascade ensues to completely close the phagosome and subsequently traffic lysosomal vesicles to the phagosome (May, 2001). First, phosphatidyl-4-phosphate 5-kinase phosphorylates phosphatidylinositol (4)-phosphate to phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2; Loijens et al., 1996), which activates phosphoinositide 3 kinase-I (PI3 K-I) in order to phosphorylate PI(4,5)P2 to phosphatidylinositol (3,4,5)-trisphosphate (PI(3,4,5)P3; Marshall et al., 2001; Greenberg and Grinstein, 2002; Vieira et al., 2002; Gu et al., 2003; Yeung et al., 2006). At this stage, AKT can tether its PH domain to PI(3,4,5)P3 (Marshall et al., 2001). Subsequently, Arp2/3-dependent actin filaments assemble by zippering around the phagosome cup (Caron, 2000; May, 2000; Greenberg and Grinstein, 2002).
Once the phagosome is formed and internalized, the lipid composition of the membrane begins to undergo changes. This is also when depolymerization of actin filaments surrounding the phagosome occurs (Scott et al., 2003, 2005; Yeung et al., 2006). A PI(3,4,5)P3 5′-lipid phosphatase, SHIP-I, dephosphorylates PI(3,4,5)P3 to form phosphatidylinositol (3,4)-bisphosphate (Damen et al., 1996). Subsequently, PI 3-kinase III (PI3 K-III) phosphorylates phosphatidylinositol (PI) to produce phosphatidylinositol (3)-phosphate (PI(3)P; Toker and Cantley, 1997). Phagosomes adopt early endosome markers (Rab5 GTPase and early endosome antigen-1) when endosomes fuse with phagosomes (Desjardins, 1995; Vieira et al., 2001, 2003; Duclos et al., 2003) and then lysosomal markers when lysosomes fuse with phagosomes to form a phagolysosome (Pitt et al., 1992; Desjardins, 1995; Tjelle et al., 2000; Vieira et al., 2002; Duclos et al., 2003). If the phagocytosed particle is a microorganism, acid hydrolases in the lysosome degrade the microorganism; however, there is no mechanism for lysosomal degradation of particles like silica and latex.
Complement receptor 3 (CR3)–mediated uptake of complement-opsonized particles such as complement-opsonized zymosan (COZ) shares some features with the FcR-mediated pathway, yet there are distinct mechanistic differences (Newman et al., 1991; Allen and Aderem, 1996; Caron and Hall, 1998). Unlike FcR-mediated uptake, efficient complement-opsonized-particle uptake by CR3 (also known as integrin Cd11b/Cd18 or Mac-1) requires cells to be primed with phorbol 12-myristate 13-acetate (PMA) to activate protein kinase C signaling (Castagna et al., 1982). Another mechanistic difference is the formation of an actin-rich cellular pedestal in the presence of complement-opsonized particles (Hall et al., 2006; Bohdanowicz et al., 2010; Lee et al., 2011). The protrusion and uptake depend on the integrity of both the actin and microtubule cytoskeleton (Patel and Harrison, 2008). Phosphorylation of Src and Syk tyrosine kinases occurs during both types of uptake (Aderem and Underhill, 1999; Shi et al., 2006), but RhoA GTPase is activated during CR3-mediated uptake rather than Rac GTPase. The phosphatidylinositol lipid signaling cascade that follows phagosome internalization (Bohdanowicz et al., 2010) is similar for CR3- and FcR-mediated uptake (Bohdanowicz et al., 2010).
In the present study, we compare the nonopsonized silica and latex particle uptake and processing pathway to the well-characterized FcR- and CR3‑mediated pathways. GTPase and PI3K activation is similar to the opsonized-particle pathways except that nonopsonized particles activate both Rac and RhoA GTPases. Unlike FcR-mediated uptake, nonopsonized-particle uptake requires both actin and microtubule cytoskeletons. In addition, GTPase and PI3K activation requires the presence of microtubules. Finally, we establish that silica particles need to be internalized to be toxic, since binding of silica to the cell surface is insufficient to damage cells. Thus nonopsonized-particle uptake has unique characteristics that distinguish it from both FcR- and CR3-mediated uptake.
RESULTS
Pharmacological inhibitors of phagocytosis
To begin to characterize the nonopsonized-particle phagocytosis pathway, we treated cells with various pharmacological reagents that have been shown to inhibit FcR- and/or CR3-mediated uptake pathways. Phosphorylation of Fc-receptor ITAM regions by Src tyrosine kinases (Lyn, Hck, Fgr) and autophosphorylation of Syk tyrosine kinase are two of the initial steps of particle recognition during FcR-mediated phagocytosis (Kiefer et al., 1998; Suzuki et al., 2000). Inhibitors of tyrosine kinases reduce uptake through this pathway but have little effect on CR3-mediated uptake (Allen and Aderem, 1996). To address the requirement for tyrosine kinase activity for nonopsonized particles, we used PP2, a broad-spectrum tyrosine kinase inhibitor (Hanke et al., 1996). Uptake of both Ab-opsonized and nonopsonized particles by macrophages treated with 20 μM PP2 was reduced by approximately 50% (Figure 1A and Supplemental Figure S1A), indicating a role for tyrosine kinases in both processes.
FIGURE 1:
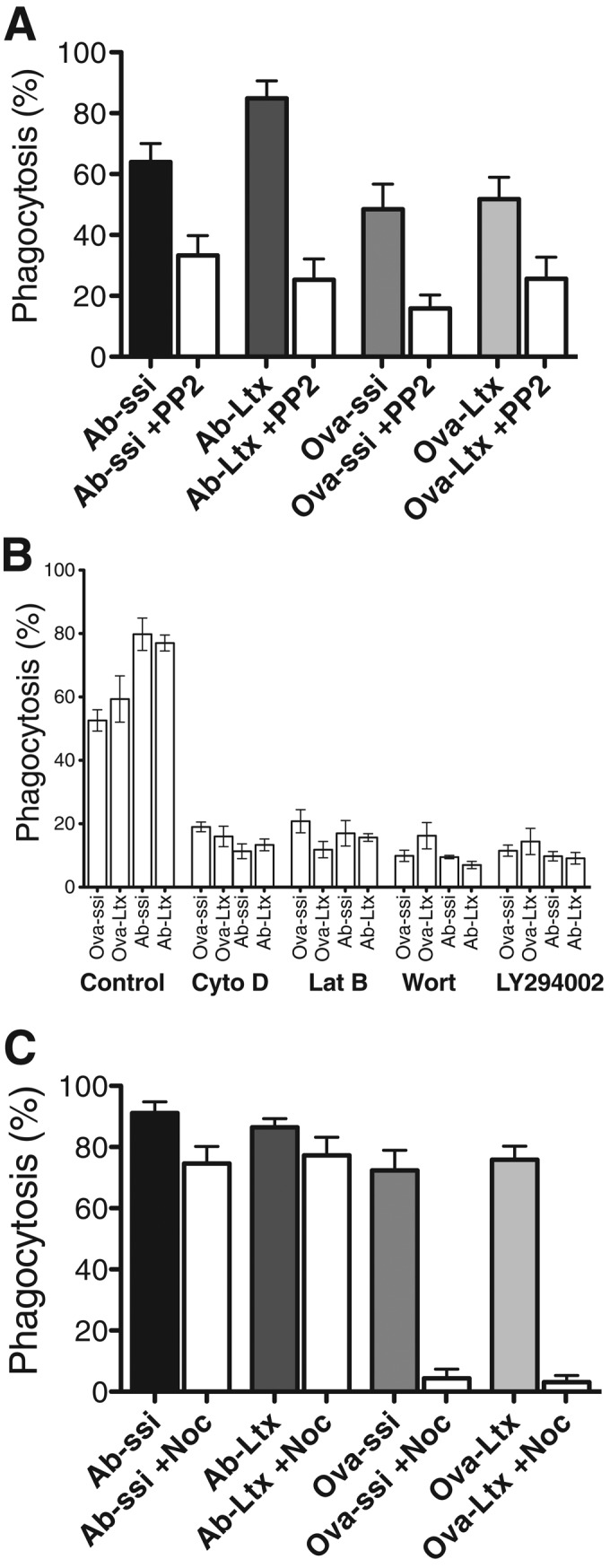
The effect of pharmacological inhibitors on particle uptake. Cells were treated with inhibitors for (A) tyrosine phosphorylation (20 μM PP2), (B) PI3 K-I (20 μM LY294002, 20 nM wortmannin [Wort]), or actin polymerization (500 nM latrunculin B [Lat-B], 500 nM cytochalasin D [Cyto-D]), or (C) microtubule polymerization (800 nM nocodazole [Noc]) and subsequently exposed to Ab-opsonized spherical silica or latex (Ab-ssi, Ab-Ltx) or nonopsonized spherical silica or latex (Ova-ssi, Ova-Ltx) particles for 45 min. The presence of inhibitor in A and B results in a decreased amount of both Ab-opsonized- and nonopsonized-particle uptake, but only nonopsonized-particle uptake in C is affected by the presence of nocodazole. Error bars represent SEM of at least three experiments performed in duplicate on separate days.
The next step of phagocytosis that is commonly observed is the activation of PI3 K-I, leading to the production of PI(3,4,5)P3 (Cox et al., 1999). Macrophages were treated with either 20 nM wortmannin or 20 μM LY294002 to inhibit PI3 K-I and then exposed to particles. Uptake of both Ab-opsonized and nonopsonized particles was significantly reduced in the presence of either compound (Figure 1B and Supplemental Figure S1B). These data suggest that PI3 K-I activation plays a similar role during both FcR-mediated and nonopsonized-particle phagocytosis.
Cytoskeletal actin filaments are polymerized to protrude the membrane and surround the particle during internalization by macrophages (Swanson, 1995; Machesky, 1999; May, 2000). To determine the role of actin filament polymerization during nonopsonized-particle uptake, we tested inhibitors of cytoskeletal filament formation. Cells were treated with either 500 nM latrunculin B or 500 nM cytochalasin D to inhibit actin polymerization before particle addition. Both drugs dramatically inhibited uptake of particles (Figure 1B and Supplemental Figure S1B), consistent with the observation of an actin-rich phagosome surrounding nonopsonized silica particles (Haberzettl et al., 2007; Costantini et al., 2011).
CR3-mediated uptake of complement-opsonized particles, but not FcR-mediated uptake of Ab-opsonized particles, depends on an intact microtubule cytoskeleton (Newman et al., 1991; Allen and Aderem, 1996). To determine whether nonopsonized-particle uptake requires microtubules, we treated cells with nocodazole before particle exposure. To minimize potential off-target effects of the compound, the minimum concentration of nocodazole necessary to depolymerize microtubules, 800 nM, was used (Supplemental Figure S2). Consistent with earlier results, Ab-opsonized-particle uptake was unaffected by nocodazole treatment (Figure 1C and Supplemental Figure S1C; Newman et al., 1991; Allen and Aderem, 1996). Surprisingly, nocodazole not only inhibited CR3-mediated uptake, but it also blocked internalization of both nonopsonized silica particles and latex beads (Figure 1C and Supplemental Figure S1C). This result indicates that nonopsonized-particle uptake is distinct from FcR-mediated uptake. It is also distinct from CR3-mediated uptake because in order for cells to take up complement-opsonized particles, they must be preactivated with PMA (Castagna et al., 1982; Allen and Aderem, 1996). Uptake of nonopsonized particles does not require PMA activation. Of interest, the fact that untreated cells cannot take up complement-opsonized COZ indicates that not all types of particles encountered by cells can be taken up by the nonopsonized uptake pathway.
Rho-family GTPases are activated during nonopsonized-particle uptake in a microtubule-dependent manner
FcR engagement with Ab-opsonized particles triggers activation of Rac1 GTPase and Cdc42, whereas binding of complement-opsonized particles to CR3 triggers activation of RhoA GTPase (Caron and Hall, 1998; Caron, 2000; Caumont et al., 1998; Honda et al., 1999). To establish which Rho-family GTPases are activated in the presence of nonopsonized particles, we exposed cells to nonopsonized particles, Ab-opsonized particles, or COZ and measured the extent of GTPase activation. Within 30 s, COZ and nonopsonized particles activated RhoA to a similar extent and with similar timing (Figure 2A), whereas Ab-opsonized particles showed little activation. Cells exposed to Ab-opsonized particles activated Rac within 30 s (Figure 2B), whereas it took 15 min to see a comparable activation of Rac with nonopsonized particles (Figure 2B). There was no significant activation of Rac by COZ. Thus, unlike FcR- and CR3-mediated phagocytosis, both Rac and RhoA GTPases are activated during nonopsonized-particle uptake.
FIGURE 2:
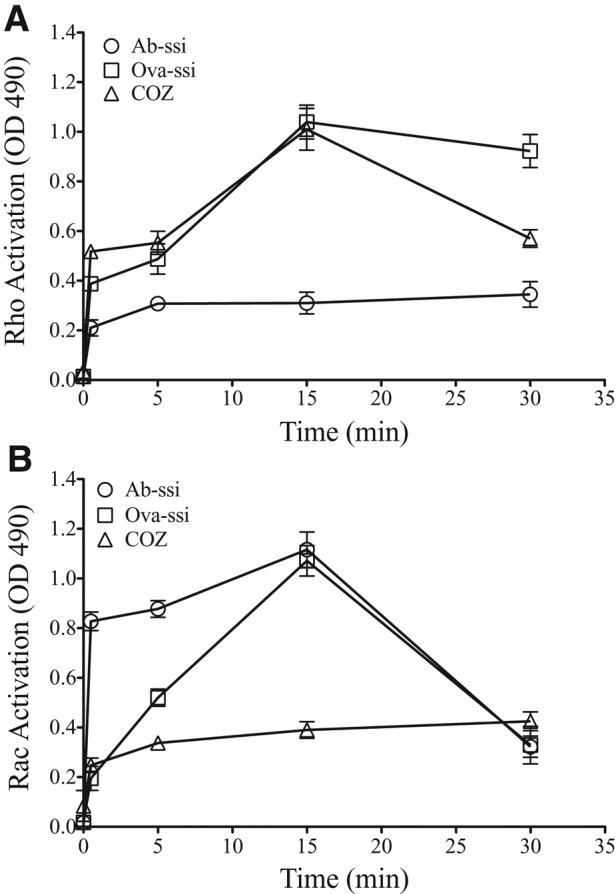
Exposure to nonopsonized particles activates RhoA and Rac GTPase. Macrophages were exposed to antibody-opsonized spherical silica (Ab-ssi; circles), ovalbumin-coated spherical silica (Ova-ssi; squares), or COZ (triangles) for increasing time points before lysate collection. The kinetics of activation of either RhoA GTPase (A) or Rac GTPase (B) was measured by HRP absorbance at 490 nm using a G-LISA fluorescence detection assay. The kinetics is slower than during other assays due to acclimation to a synchronization temperature. Maximum activation of both Rac and RhoA occurs by 15 min after exposure. Only nonopsonized particles elicit both RhoA and Rac GTPase activation. Error bars represent SEM of two experiments performed in duplicate on two separate days.
Because nocodazole inhibited the uptake of nonopsonized particles, it was important to determine the stage at which this inhibition occurs. To establish whether microtubules are required before GTPase activation, we treated cells with nocodazole and then exposed them to particles for 15 min. Nocodazole inhibited RhoA and Rac activation in cells exposed to nonopsonized particles (Figure 3, A and B) and inhibited RhoA activation in cells exposed to COZ particles (Figure 3A). As expected, the depolymerization of microtubules did not affect the activation of Rac in cells exposed to Ab-opsonized particles (Figure 3B). Thus microtubules seem to be involved at an early step in the process of phagocytosis of COZ and nonopsonized particles.
FIGURE 3:
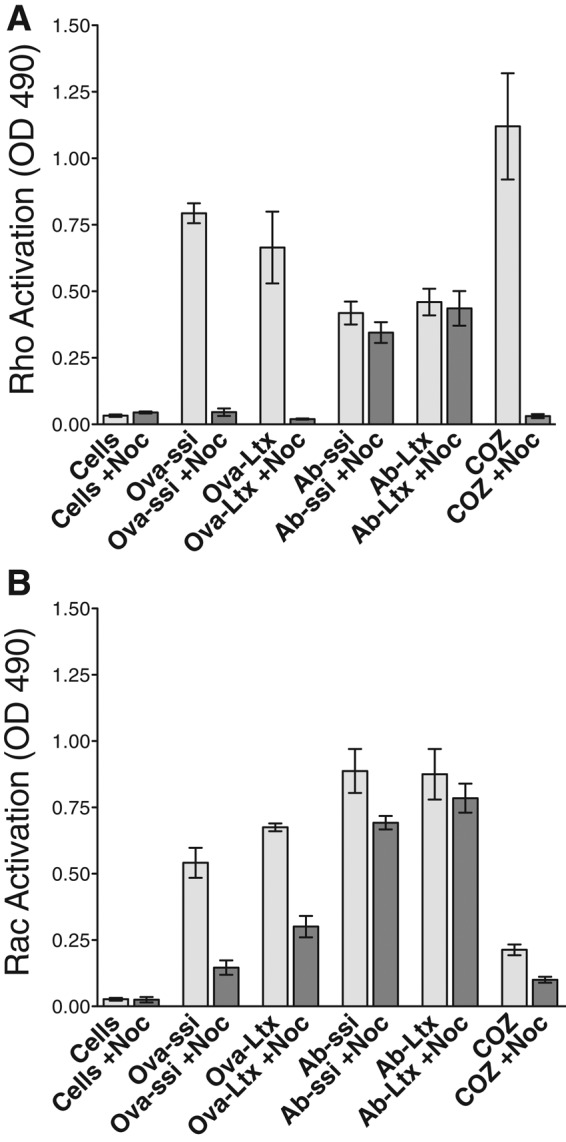
Neither RhoA nor Rac is activated upon nonopsonized particle exposure in the presence of nocodazole. Cells were treated with 800 nM nocodazole (Noc) and subsequently exposed to no particles (Cells), nonopsonized particles (Ova-ssi, Ova-Ltx), antibody-opsonized particles (Ab-ssi, Ab-Ltx), or COZ for 15 min. Activation of RhoA (A) or Rac (B) GTPase was measured by HRP absorbance at 490 nm using a G-LISA fluorescence detection assay. In the presence of Noc (dark gray bars), RhoA and Rac GTPase activation are diminished when cells are exposed to Ova-ssi, Ova-Ltx, or COZ but not Ab-ssi or Ab-Ltx. Error bars represent SEM of two experiments performed in duplicate on two separate days.
Silica is cytotoxic to macrophages and activates both Rac and RhoA GTPase. It is possible that activation of both GTPases is an important part of the pathway that leads to silica-induced cell death. To test this hypothesis, we exposed cells to nonopsonized or Ab-opsonized 3-μm latex beads, which are not toxic to cells, and measured the activation of GTPases. The results were similar to those obtained with silica particles. Nonopsonized latex beads activated both RhoA and Rac GTPases, whereas Ab-opsonized latex beads showed greater Rac activation. In the presence of nocodazole, the GTPases were not activated by nonopsonized particles but were activated by Ab-opsonized particles (Figure 3, A and B). Therefore simultaneous activation of both Rac and RhoA is not sufficient to cause cell death. In addition, the data show that the dual GTPase activation is not related to some specific characteristic of silica particle surface chemistry.
PI(3,4,5)P3 accumulation on nonopsonized particle phagosomes is a microtubule-dependent process
The data in Figure 1 indicate that PI3 K-I activation is required for Ab-opsonized- and nonopsonized-particle uptake. To determine whether the spatial and temporal dynamics of PI(3,4,5)P3 accumulation at sites of phagocytosis differ between Ab-opsonized and nonopsonized particles, we imaged cells expressing AKT-PH–green fluorescent protein (GFP) during phagocytosis. When these cells were exposed to Ab-opsonized or nonopsonized particles, AKT-PH-GFP rapidly accumulated around both types of nascent phagosomes (Figure 4, A and B). Before particles were completely internalized, the probe dissociated from the phagosome. The extent of accumulation and the time course of association and dissociation were similar for Ab-opsonized and nonopsonized silica particles (Figure 4D), as well as 3-μm (Ab-opsonized and nonopsonized) latex beads (unpublished data), suggesting that all particle types activate PI3 K-I equivalently. When cells were treated with either wortmannin or LY294002 before particle addition, no nascent phagosomes were formed, and AKT-PH-GFP did not accumulate at sites where particles were bound to the cell (unpublished data). To test whether microtubules are required for PI3K activation, we treated macrophages transiently expressing AKT-PH-GFP with nocodazole and then exposed them to silica particles. AKT-PH-GFP accumulated at Ab-opsonized-particle phagosomes (unpublished data) but did not accumulate at sites where nonopsonized particles were bound to cells (Figure 4C), suggesting that PI3 K-I activation also depends on the presence of microtubules during nonopsonized-particle uptake.
FIGURE 4:
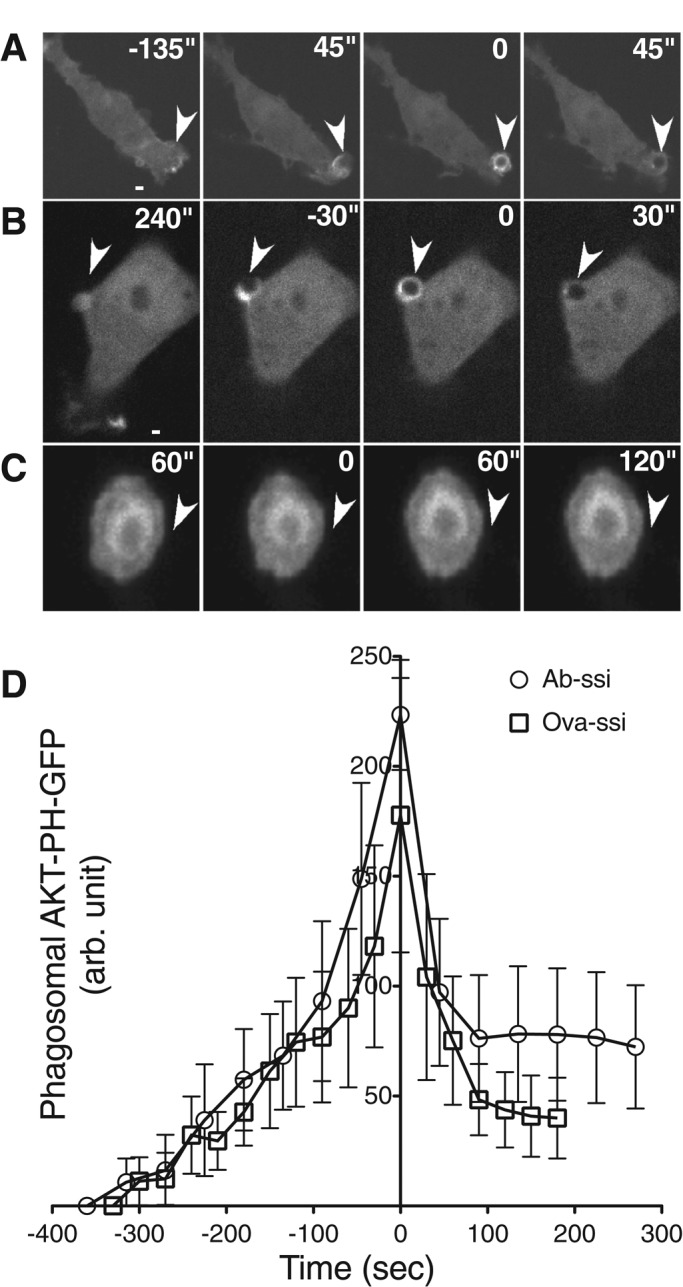
PI(3,4,5)P3 concentrates at nonopsonized-particle phagosomes but not in the presence of nocodazole. Cells were transiently transfected with AKT-PH-GFP to detect accumulation of PI(3,4,5)P3 at particle phagosomes. When exposed to (A) Ab-opsonized spherical silica or (B) ovalbumin-coated spherical silica, AKT-PH-GFP quickly concentrates at the site of the particle and dissociates once a particle has been internalized. (C) When cells are treated with 800 nM nocodazole, AKT-PH-GFP does not accumulate around nonopsonized- particle phagosomes. These data are representative of localization visualized on six separate days. (D) The rate of AKT-PH-GFP association with and dissociation from particle phagosomes. N = 12. Time zero represents maximum localization after particle–cell interaction, and error bars represent SEM.
Actin polymerization during particle phagocytosis is a microtubule-dependent process
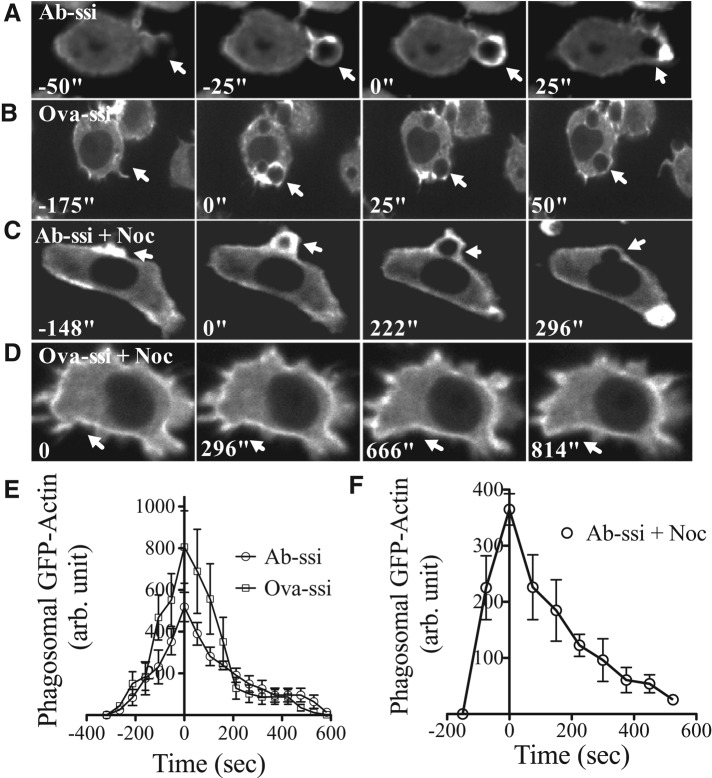
The kinetics of F-actin accumulation around Ab-opsonized particles during Fc receptor–mediated phagocytosis is well characterized (Swanson, 1995; Machesky, 1999; May, 2000). To study F-actin dynamics during nonopsonized-particle phagocytosis, we exposed macrophages stably expressing GFP-actin to nonopsonized or Ab-opsonized particles. Actin accumulates around both particle types at a similar rate and to a similar extent during uptake (Figure 5, A, B, and E). Once particles are internalized, actin dissociates from both types of phagosomes at a similar rate. Actin-rich pseudopods also accumulate around COZ particles, but only when cells were stimulated with PMA before particle addition (Supplemental Figure S3). Without PMA treatment, no actin response was observed, and there was no uptake of particles. Further, when PMA-treated cells were exposed to zymosan that was not complement opsonized, there was no actin localization and no uptake (unpublished data).
FIGURE 5:
Actin-rich protrusions do not extend around nonopsonized-particle phagosomes when microtubules are depolymerized. GFP-actin macrophages were exposed to either (A) Ab-opsonized or (B) nonopsonized particles and imaged to determine the time course of actin ring association with particle phagosomes. Actin-rich phagosomes form around, and dissociate from, Ab-opsonized and nonopsonized particles on a similar time scale. When cells were treated with 800 nM nocodazole, actin associated with Ab-opsonized-particle phagosomes (C) but not nonopsonized-particle phagosomes (D). (E, F) The time course of actin association with and dissociation from particle phagosomes is similar when cells are exposed to either Ab-opsonized or nonopsonized particles. N = 40. Time zero represents maximum localization after particle–cell interaction. (F) The time course of actin association with and dissociation from Ab-opsonized particle phagosomes in the presence of nocodazole. Time zero represents maximum localization after particle–cell interaction. N = 4. Error bars represent SEM.
We have established that the microtubule network is necessary for RhoA and Rac GTPase activation, as well as for PI3 K-I activation. To determine whether the presence of microtubules also affects actin accumulation at sites of phagocytosis, we treated cells expressing GFP-actin with nocodazole and then exposed them to nonopsonized or Ab-opsonized silica or COZ. F-actin localized around Ab-opsonized particles with kinetics similar to untreated cells (Figure 5, C and F, and Supplemental Video S1). No localization of the actin probe was observed at sites where nonopsonized particles (Figure 5D and Supplemental Video S2) or COZ particles were bound to cells (unpublished data).
We noticed that when cells were treated with nocodazole, the GFP-actin probe rapidly accumulated in the peripheral cell cortex (Figure 5, C and D, and Supplemental Figure S4, A and C). When cells were treated with nocodazole and subsequently fixed and stained with rhodamine phalloidin, there was an increase in the total cortical F-actin as well (Supplemental Figure S4, B and D). This result indicates that there is a general change in the structure of the cortical cytoskeleton in response to microtubule depolymerization. This change may provide a clue as to why cells without intact microtubules are unable to phagocytose some types of particles.
To better understand whether F-actin polymerization contributes to Ab-opsonized- and nonopsonized-particle phagosome initiation and maturation to a similar extent, we treated stably transfected GFP-actin macrophages with either cytochalasin D or latrunculin to inhibit actin polymerization and then exposed them to either Ab-opsonized or nonopsonized silica. In the presence of cytochalasin D, both particle types formed punctate actin spots (Supplemental Figure S5A). In the presence of latrunculin, cells exposed to Ab-opsonized silica formed a punctate spot at the site of particle binding, but the cup never surrounded the particle, whereas cells exposed to nonopsonized silica did not show any actin response at all (Supplemental Figure S5B).
Because microtubules are necessary for nonopsonized-particle uptake, it is possible that surface-bound particles might be internalized after repolymerization of microtubules. Nonopsonized particles were allowed to bind to cells in the presence of nocodazole for 45 min, and then microtubules were allowed to repolymerize by changing the cells to fresh medium without nocodazole. Within 5 min of nocodazole washout, microtubules had repolymerized (unpublished data). Nonopsonized silica particles that were attached to the cells were not internalized over the next 2 h (Supplemental Figure S6, arrowhead). However, nonopsonized latex particles added to the cells after nocodazole was removed were internalized within 10 min (Supplemental Figure S6, arrow). The same phenomenon was observed when the order of addition of the particles was reversed (unpublished data). These data indicate that the presence of microtubules is not sufficient to drive phagocytosis of nonopsonized particles, but instead that a dependent sequence of events appears to be required.
Endolysosomal vesicle fusion with phagosomal membranes occurs once actin dissociates from particle phagosomes
As FcR-mediated phagosome maturation progresses, membrane is contributed to the nascent phagosome from endosomal and lysosomal vesicles (denoted as endolysosomal vesicles) fusing with phagosomes (Bajno et al., 2000). GFP-actin–expressing macrophages were incubated with red fluorescent dextran for 90 min to label endolysosomal compartments and then exposed to silica particles. The phagosomes gradually increased in fluorescence intensity after the particles were internalized (Figure 6A and Supplemental Video S3). The actin associated with phagosomes disappears before detection of an increase in dextran fluorescence, indicating that it is the internalized, actin-free phagosome to which the endolysosomal vesicles fuse. The time course and extent of increase in fluorescence intensity due to endolysosomal vesicle fusion were similar for Ab-opsonized and nonopsonized silica particles (Figure 6B).
FIGURE 6:
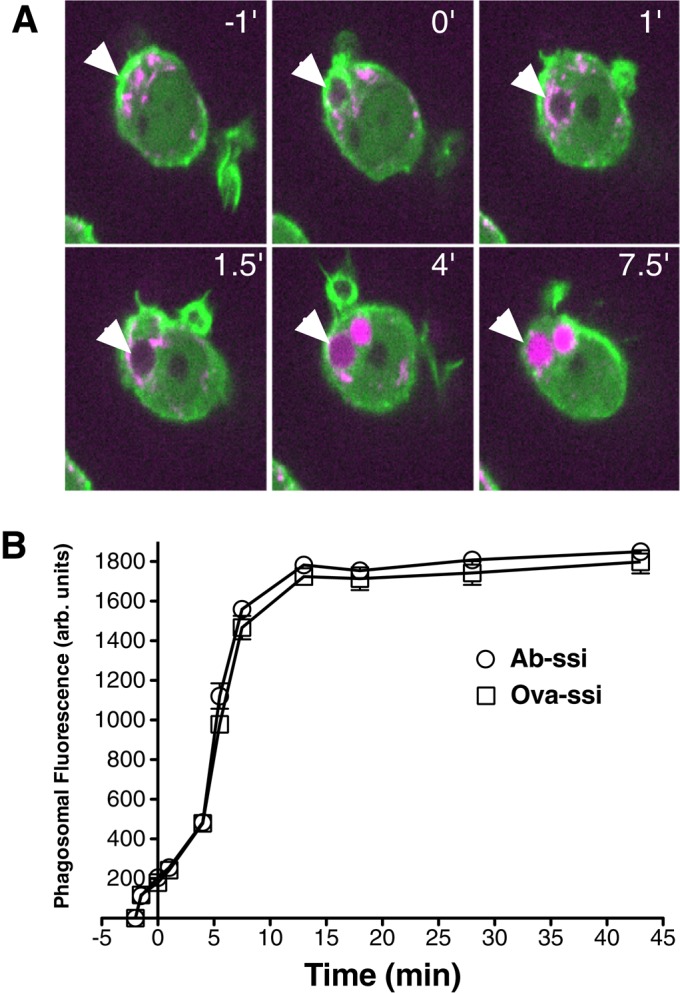
Actin dissociation precedes endolysosomal vesicle fusion with phagosomal membranes. (A) GFP-actin macrophages were preloaded with a red fluorescent dextran before particle addition. Actin rings (green) formed around nonopsonized particles (arrowheads) and then dissociated rapidly after internalization. Phagosomes then increased in red fluorescence intensity due to endolysosome fusion (shown in magenta). (B) Quantification of phagosomal dextran fluorescence for opsonized and nonopsonized silica particles. N = 30. Time zero represents the time when the actin ring first begins to form. Error bars represent SEM.
Appearance of PI(3)P around nonopsonized particle phagosomes
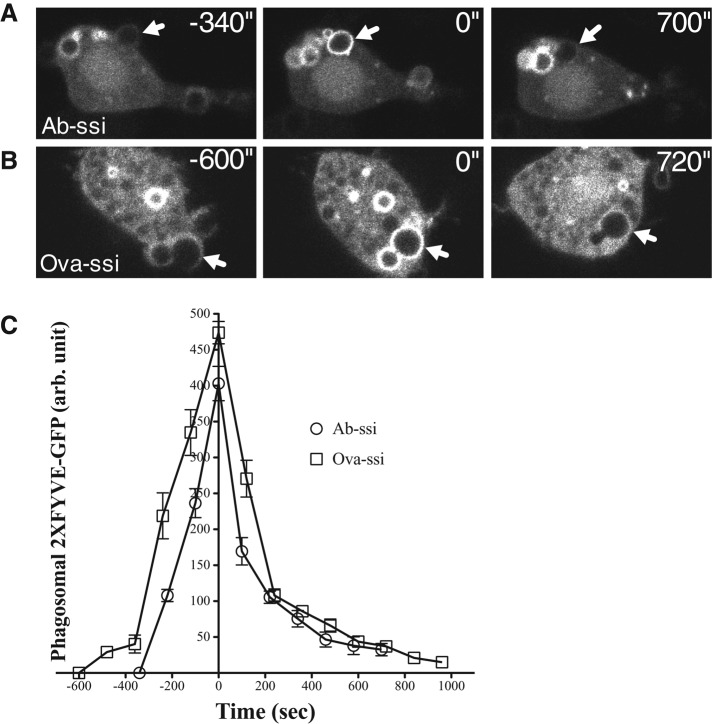
After endosomes fuse with phagosomes, PI(3)P can be detected in association with the phagosomal membrane (Vieira et al., 2001). To determine whether the vesicle trafficking pathway reaches this step during nonopsonized-particle phagosome maturation, we exposed macrophages transiently expressing 2xFYVE-GFP, a probe for PI(3)P, to either Ab-opsonized or nonopsonized spherical silica particles. The probe was recruited similarly to the membranes surrounding both particle types (Figure 7), suggesting that both undergo the same vesicle trafficking events once taken up into cells. Cells transiently cotransfected with Ruby-Lifeact and 2xFYVE-GFP show that actin associates with both Ab-opsonized- and nonopsonized-particle phagosomes before 2xFYVE-GFP associates with the phagosome (Supplemental Video S4). Sometimes the Lifeact signal is completely lost from phagosomes before 2xFYVE-GFP appears, and sometimes the two signals overlap. These results indicate that the later events in vesicle trafficking are similar for opsonized and nonopsonized phagosomes.
FIGURE 7:
PI(3)P tethers to nonopsonized-particle phagosomes. Cells were transiently transfected with vector expressing 2xFYVE-GFP to detect accumulation of PI(3)P. When cells were exposed to (A) Ab-opsonized or (B) nonopsonized spherical silica, 2xFYVE-GFP accumulates at phagosomes within 6 min relative to initial particle–cell interaction and persists long after the particle has been internalized. (C) Quantification of the association of 2xFYVE-GFP with phagosomes. N = 6. Time zero indicates maximum accumulation of 2xFYVE-GFP at particle phagosomes. 2xFYVE-GFP accumulates at and dissociates from opsonized and nonopsonized particle phagosomes at a similar rate. Error bars represent SEM.
PI3 kinase-I activation, actin polymerization, and microtubule polymerization are necessary for silica-induced cell death in macrophages
Because cells bind but do not internalize particles in the presence of several pharmacological inhibitors, we were able to address the question of whether silica toxicity requires particle internalization or, alternatively, whether particle surface binding is sufficient to induce cell death. In the absence of inhibitors, 50–100% of the cells die within 10 h when macrophages are exposed to nonopsonized silica particles (Gilberti et al., 2008). Cells were treated with silica particles plus concentrations of wortmannin, LY294002, cytochalasin D, latrunculin, or nocodazole that inhibit phagocytosis but are not toxic to cells (Supplemental Figure S7). The inhibitor-treated cells had an equivalent number of surface-bound particles as the untreated cells normally internalize (unpublished data); however, the surface-bound particles were not toxic (Figure 8). These data suggest that particle internalization is necessary to induce cell death.
FIGURE 8:
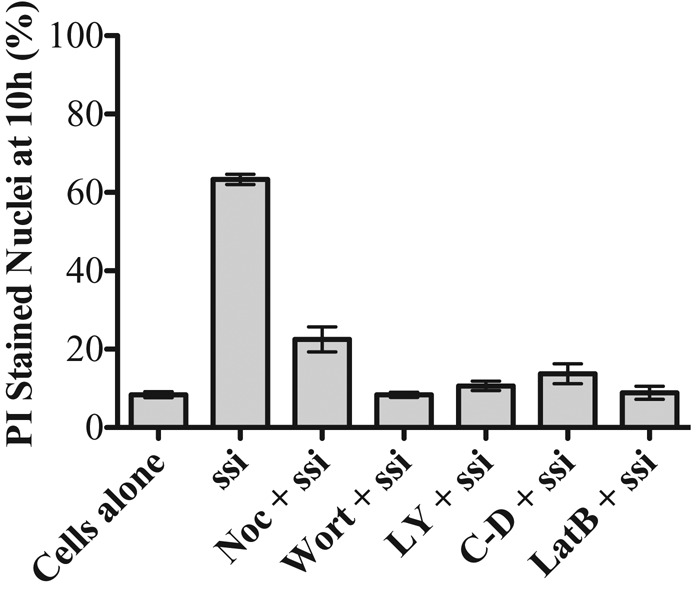
Phagocytosis is necessary to trigger silica-induced cell death in macrophages. Cells were treated with nocodazole (Noc), cytochalasin D (C-D), latrunculin B (LatB), LY294002 (LY), or wortmannin (wort), at the concentrations used to inhibit phagocytosis, in the presence of propidium iodide to measure the extent of membrane-permeable cells at 10 h. Nonopsonized, ovalbumin-coated 3 µm spherical silica (ssi) particles are only toxic to cells in the absence of inhibitor. Error bars represent SEM.
DISCUSSION
When a particle interacts with a cell surface, a transmembrane receptor typically recognizes the particle and initiates phagocytosis. For Ab- and complement-opsonized particles, the receptors and their interaction with particles have been extensively studied. However, whether there is a specific cell-surface molecule that interacts with nonopsonized particles is unclear. There is evidence that scavenger receptor family-A receptors (SR-A) may play a role in recognizing negatively charged silica particles (Taylor et al., 2005); however, uptake of silica also occurs in SR-A–deficient cells (Hamilton et al., 2006; Thakur et al., 2009), and there is no reason to expect SR-A to interact with latex particles, which are also readily internalized. More important, we found no difference in the uptake of silica coated with proteins of various charge or of latex beads that have negative, positive, or neutral surface charges (Gilberti et al., 2008). Furthermore, uptake occurs similarly whether particles are uncoated or ovalbumin coated (Costantini et al., 2011). There is no reason to suspect that cells have evolved specific uptake machinery for a particle surface that macrophages rarely encounter. We hypothesize that nonopsonized particles are internalized by a nonspecific mechanism involving surface charge interaction. There are sufficient mobile charged molecules in the plasma membrane for us to presume that local charges can rearrange to allow for a stable particle–cell interaction.
Once particles are bound, cells seem to nonspecifically activate the phagocytosis machinery. This normally occurs due to receptor clustering, but it appears that nonspecific binding can activate normal phagocytosis. In our model, receptor clustering at the site of nonspecific binding triggers phagocytosis. This is consistent with the fact that FcR expression in FcR-deficient COS-7 cells allows for efficient phagocytosis of Ab-opsonized particles in a manner similar to macrophages, which normally express FcR (Indik et al., 1991). There are notable differences in the uptake machinery of FcR- and CR3-mediated uptake. FcR-mediated uptake is independent of macrophage activation and the presence of microtubules (Newman et al., 1991; Allen and Aderem, 1996). CR3-mediated uptake requires activation of macrophages by PMA (Castagna et al., 1982) and is dependent on microtubules (Newman et al., 1991; Allen and Aderem, 1996). We have now established the events that occur in nonspecific particle phagocytosis once binding occurs, regardless of the mechanism of binding.
To investigate the molecular pathway of nonopsonized-particle phagocytosis, a variety of markers were chosen that have been well characterized for FcR- and CR3-mediated uptake. We considered the possibility that some difference in the uptake and vesicle trafficking pathway for nonopsonized particles might play an important role in silica particle toxicity. Our results show that, for the most part, the opsonized and nonopsonized pathways share many properties. More important, nonopsonized silica (toxic) and nonopsonized latex (nontoxic) were indistinguishable with the probes we examined. There are interesting differences between nonopsonized- and opsonized-particle uptake. Nonopsonized particles activate both RhoA and Rac GTPase, whereas only Rac is activated during FcR-mediated uptake, and only RhoA is activated during CR3-mediated uptake (Figure 2). Although it is possible that dual GTPase activation might relate to the toxicity of nonopsonized silica, this seems unlikely since similar results were obtained upon exposure to nonopsonized, nontoxic latex beads, suggesting that this is a characteristic of nonopsonized-particle uptake. Thus it does not appear that silica toxicity is related to a particular pathway associated with nonopsonized-particle uptake.
The most important difference in the uptake pathway of nonopsonized particles is that, like complement-opsonized particles, nonopsonized-particle uptake depends on microtubules. Depolymerization of microtubules blocks particle uptake and toxicity (Figures 1 and 8 and Supplemental Figure S2). However, whereas CR3-mediated uptake requires macrophages to be primed with PMA, nonopsonized phagocytosis does not require preactivation. Of interest, non–complement-opsonized yeast or zymosan particles are not internalized even if cells are initially primed. These data suggest that not all particle types that cells encounter are susceptible to nonspecific, nonopsonized-particle phagocytosis. Whether it is the microtubules themselves or something associated with the microtubule that is required for phagocytosis remains unclear. We have been unable to image individual microtubules in macrophage cell lines, so we cannot say whether there is an altered accumulation of microtubules at sites of phagocytosis. Evidence indicates that CLIP-170 plays an important role in CR3-mediated phagocytosis through its interaction with mDia (Lewkowicz et al., 2008), so it is likely that a similar interplay of microtubules, microtubule-associated proteins, and actin nucleation factors is required for nonopsonized-particle phagocytosis.
In the presence of nocodazole, nonopsonized particles do not stimulate a significant Rac or RhoA GTPase response (Figure 3), and there is no PI(3,4,5)P3 or F-actin localization at the site of particle–cell interaction (Figures 4 and 5). Thus microtubules are needed to initiate the phagocytosis machinery from the earliest known part of the pathway. To further elucidate the role of microtubules during uptake, we treated GFP-actin–expressing macrophages with nocodazole, exposed them to nonopsonized particles that bound to the cells, and then removed nocodazole from the culture media to allow microtubules to regrow. When the cells were then exposed to new, nonopsonized particles, only the particles that were added after removing nocodazole were internalized (Supplemental Figure S6). The particles initially added to the cells were still bound to the cells but never internalized. These data suggest that when a particle binds to a cell, a signal transduction cascade is initiated that requires microtubules. The initial activation event then likely becomes attenuated so that even when microtubules are repolymerized, the activation signal is not sent, and the particles are never taken up.
Another interesting effect of nocodazole treatment is a change in overall F-actin distribution in cells (Supplemental Figure S4). Rapid accumulation of F-actin around the cell periphery occurs when microtubules are depolymerized. This result is consistent with evidence for interaction between the microtubule and actin cytoskeletons (Bartolini and Gundersen, 2010; Zhou et al., 2010a, b; Dent et al., 2011). In the absence of microtubules, cells may lose control of specific sites of actin polymerization and/or depolymerization, leading to wholesale accumulation of F-actin around the periphery of the cell. Thus it is possible that the lack of uptake may be due to an overabundance of F-actin in the cytoskeleton rather than an inability to polymerize actin at a specific site. However, it should be noted that Ab-opsonized particles are still taken up under these conditions.
Even though these studies have provided insight into how nonopsonized particles are internalized, they do not directly inform us about a crucial aspect of the process—how silica particles induce cell death. Leakage from phagolysosomal compartments is the earliest known event in silica-induced cell death (Thibodeau et al., 2004; Costantini et al., 2011; Joshi and Knecht, 2013). Leakage occurs within 90 min after particle exposure, and cell death occurs many hours later. Our initial hypothesis was that some aspect of signaling or vesicle processing might be altered in silica phagocytosis, and this might make phagosomes particularly sensitive to reactive oxygen or other factors that could lead to lysosomal membrane damage (Thibodeau et al., 2004; Costantini et al., 2011; Joshi and Knecht, 2013). However, leakage occurs with nonopsonized silica but not latex particles (unpublished data), even though both appear to be taken up by the same pathway. Our results suggest that nonopsonized uptake of silica and latex are equivalent, indicating that the difference in toxicity is due to some aspect of the chemistry of the silica particle itself. This is consistent with measurements of increased reactive oxygen species (ROS) in cells treated with silica particles (Blackford et al., 1994; Vallyathan and Shi, 1997; Fubini, 1998; Huaux, 2007). Our observation that silica particles need to be internalized to be toxic (Figure 8) supports the hypothesis that if ROS is the culprit, the focus should be on phagosomal ROS formed during the respiratory burst associated with macrophage phagocytosis.
MATERIALS AND METHODS
Chemicals
All chemicals are from Sigma Chemical (St. Louis, MO), unless otherwise noted.
Tissue culture
The mouse alveolar macrophage cell line MH-S (ATCC CRL-2019) was cultured at 37°C with 5% CO2 in RPMI-1640 medium (Media Tech, Pittsburgh, PA) supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 55 μM 2-mercaptoethanol, 10% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA), 100 μg/ml ampicillin, and 100 μg/ml dihydrostreptomycin sulfate.
Particles
The particles used were amorphous 3-μm spherical silica (Allsphere silica; Alltech Associates, Deerfield, IL), zymosan A (Sigma Chemical), and 3-μm plain latex beads (Polysciences, Warrington, PA). Ovalbumin-coated (nonopsonized) particles were made by incubating 2 mg of spherical silica or 100 μl of latex beads in 1 ml of 10 mg/ml ovalbumin in particle-coating buffer (1.8 mM Na2CO3, 3.2 mM NaHCO3, 135 mM NaCl, pH 9.5) for 90 min at 37°C while tumbling. Ab-opsonized silica and latex beads were made by incubating the ovalbumin-coated particles with 20 μg/ml rabbit anti–chicken egg albumin (Immunology Consultants Laboratory, Newberg, OR) in 1 ml of phosphate-buffered saline (PBS) for 90 min at 37°C while tumbling. COZ particles were made by incubating 2 mg of zymosan A with 1 ml of non–heat-inactivated FBS for 90 min at 37°C while tumbling. All particles were stored at 4°C for a maximum of 1 mo and centrifuged and washed three times in 1× sterile PBS before addition to cells.
Transfection of MH-S macrophage cells
MH-S macrophages were plated in a 35-mm WillCo (WillCo Wells, Amsterdam, Netherlands) or ibidi (ibidi, Munich, Germany) dishes in 1 ml of RPMI-1640 complete medium at 37°C with 5% CO2 and allowed to adhere overnight. The next day, cells were transiently transfected with FuGeneHD transfection reagent (Promega, Madison, WI) and 2 μg of DNA in complete medium according to manufacturer's guidelines and allowed to incubate at 37°C overnight. Fluorescent cells were used within 24–48 h. Cells were also transiently cotransfected with Ruby-Lifeact (Riedl et al., 2008) and either AKT-PH domain fused to enhanced GFP (AKT-PH-GFP; Varnai and Balla, 1998) or 2xFYVE domain fused to green fluorescent protein (2xFYVE-GFP; Vieira et al., 2001). To examine actin dynamics during phagocytosis, cells were transfected by electroporation with a pEF6-GFP-actin probe, and a stably expressing clone was picked after selection of transformants with 3 μg/ml blasticidin (Scott et al., 2005). To maintain the stable cell line, fluorescent colonies were manually picked after limiting dilution cloning.
Time-lapse confocal microscopy
Stable GFP-actin MH-S macrophages expressing fluorescent reporter probes were plated at 1 × 105 cells/cm2 in ibidi or WillCo dishes in 1 ml of RPMI-1640 complete medium and allowed to adhere overnight. Medium was then replaced with 1 ml of CO2-independent medium (Invitrogen, Carlsbad, CA), and cells were maintained at 37°C in an ambient atmosphere incubator for 15 min before being moved to the microscope stage, where they were maintained at 37°C in a LiveCell chamber (Pathology Devices, Westminster, MD). If cells were to be transiently transfected, they were plated at a lower density so that they would be at the same density of 1 × 105 cells/cm2 for the microscopy assay. The same dish was used for transfection and subsequent imaging. Cells were transiently transfected in an ibidi or WillCo dish following the same protocol as before and remained in the dish in complete medium overnight. Medium was replaced with 1 ml of CO2-independent medium, and cells were maintained at 37°C in an ambient atmosphere incubator for 15 min before being moved to the microscope stage, where they were maintained at 37°C in a LiveCell chamber.
Cells that would be exposed to COZ were treated with 100 nM PMA in CO2-independent medium for 30 min at 37°C in an ambient atmosphere incubator. Time-lapse imaging was initiated, and then 15 μg/cm2 silica, 40-μl latex beads, or 8 μg/cm2 COZ was added to the cells. All three concentrations yield about 5 particles/cell. This concentration of silica is toxic to macrophages but also provides a low number of particles per cell to analyze phagocytosic processes.
Using a motorized XYZ stage, multiple fields of view and multiple Z-planes were captured over time. Differential interference contrast (DIC) and confocal fluorescence images were acquired using a Clara Interline charge-coupled device camera on an Andor spinning disk confocal microscope (Andor Technology, Belfast, Northern Ireland) or a Nikon A1R laser scanning confocal microscope (Nikon, Melville, NY). Particles were not all internalized in a single plane, so the most representative slice was chosen from the stack for each particle. Images were processed using ImageJ (National Institutes of Health, Bethesda, MD) to measure the increase and decrease in probe localization during uptake. Image stacks were also converted to QuickTime movies (Apple, Cupertino, CA) to observe time-lapse fluorescent protein localization and particle internalization. To measure the timing of actin, PI(3,4,5)P3, and PI(3)P association with and subsequent dissociation from particle phagosomes, a circle was drawn around the circumference of the particle at the initial location of particle–cell interaction. Mean fluorescence intensity was determined as the GFP probe associated with and subsequently dissociated from the particle phagosome over time. Time zero represents the maximum intensity of fluorescent probe association with a particle, except for panels where no uptake occurred.
To measure the increase in GFP-actin or rhodamine phalloidin stain at the cell cortex upon nocodazole treatment, a rectangle was drawn in ImageJ to vertically extend from outside to inside the cell cortex, and the mean fluorescence intensity was measured.
Quantitative phagocytosis assay in the presence of inhibitors of phagocytic processes
Phagocytosis assays were performed as described previously (Gilberti et al., 2008) except for the addition of pharmacological inhibitors before particle addition. Briefly, cells were plated at 1 × 105 cells/cm2 on 22-mm-square glass coverslips in 30-mm dishes and allowed to adhere overnight. Medium was replaced with 1 ml of CO2-independent medium for 15 min and then left untreated or treated with tyrosine kinase phosphorylation inhibitor (20 μM PP2), phosphatidylinositol 3-kinase inhibitors (20 μM LY294002 or 20 nM wortmannin), actin polymerization inhibitors (500 nM latrunculin A, 500 nM latrunculin B, or 500 nM cytochalasin D), or microtubule polymerization inhibitor (800 nM nocodazole) for 45 min at 37°C in an ambient atmosphere incubator. Cells were then exposed to particles in the presence of inhibitor for 45 min before being fixed with 4% formaldehyde for 6 min at room temperature. Formaldehyde-containing medium was removed, and 1 ml of 50 mM NH4Cl was added to quench the reaction. After fixation, the cells were stained with rabbit anti-ovalbumin antibody (Immunology Consultants Laboratory) and then fluorescein isothiocyanate–conjugated goat anti-rabbit antibody in PBS (Jackson ImmunoResearch, West Grove, PA). Particles that were opsonized with anti-ovalbumin antibody (Ab-silica and Ab-latex beads) were stained with secondary antibody only. Imaging was performed with a 63× oil immersion objective on a Zeiss Axiovert 200M microscope (Zeiss, Göttingen, Germany) using a Hamamatsu ORCA-ER camera (Hamamatsu, Bridgewater, NJ).
The surface-bound particles were counted from the fluorescence images, and the total number of particles from the DIC images. At each time point, the ratio of internalized particles to total particles was used to determine the uptake efficiency. In addition, the phagocytic index was determined as the number of internalized particles per cell per 100 macrophages. The error bars depict SE from three separate experiments.
RhoA and Rac GTPase activation
Cells were plated at 3 × 105 cells/ml in 35-mm tissue culture dishes in 1 ml of RPMI-1640 complete medium and allowed to adhere overnight, after which medium was changed to 500 μl of CO2-independent medium for 15 min. Cells that would be exposed to COZ were treated with 100 nM PMA as previously described. All dishes were chilled on ice, and then 15 μg/cm2 nonopsonized or Ab-opsonized 3-μm spherical silica or 8 μg/cm2 COZ was added. The dishes were then centrifuged at 4°C at 300 × g for 5 min to spin the particles onto the cells. Before placing the dishes back in the 37°C ambient air incubator, we added 500 μl of 37°C prewarmed CO2-independent medium to the dishes to accelerate the process of warming the cells back to 37°C. Then, cells were allowed to internalize particles for 0.5, 5, 10, 15, or 30 min before samples were lysed with G-LISA lysis buffer.
Lysates were processed for the G-LISA assay according to manufacturer's guidelines (Cytoskeleton, Denver, CO). To assay for combined Rac1, 2, and 3 (referred to as Rac) or RhoA GTPase activation, 0.3 mg of total lysate, in an equal volume of binding buffer, was added in duplicate to wells of a G-LISA plate and incubated at 4°C for 30 min. The wells were washed, and anti-Rac or anti-RhoA primary antibody was added and the plate was incubated at room temperature for 45 min. The wells were then washed and incubated with horseradish peroxidase (HRP)–labeled secondary antibody for 45 min at room temperature. HRP signal was detected at 490 nm using a multiwell spectrophotometer (SpectraMax M2; Molecular Devices Sunnyvale, CA).
Measurement of endolysosomal fusion with phagosomes
Cells were plated overnight in an imaging dish as previously described. The next day, the medium was replaced with fresh RMPI-1640 complete medium containing 1 mg/ml 70-kDa tetramethylrhodamine isothiocyanate (Sigma Chemicals) or 10-kDa Texas Red (Life Technologies) dextran and incubated for 90 min at 37°C with 5% CO2 to load the internal vesicle compartments with dextran. Medium was gently removed, and cells were washed five times with CO2-independent medium. Once cells were on the microscope stage, particles were added to the dish and allowed to settle onto the cells. Particle types included 15 μg/cm2 spherical silica particles, either ovalbumin coated or Ab opsonized. Cells were imaged using the Nikon A1R laser scanning confocal microscope every 30 s for at least 1 h to capture events of particle phagocytosis. The delivery of dextran to the phagosome due to fusion of endosomal and lysosomal vesicles was measured using ImageJ by outlining the vesicles containing the particle and measuring the increase in mean pixel intensity of fluorescent dextran over time. Image stacks were also converted to QuickTime movies.
Anti-tubulin immunostaining assay
Cells were plated on 22-mm glass coverslips in 35-mm tissue culture plastic dishes in 1 ml of RPMI-1640 complete medium and allowed to adhere overnight, after which medium was replaced with 1 ml of CO2-independent medium for 15 min at 37°C in an ambient atmosphere incubator. Next cells were fixed and immunostained using a modification of the procedure of Yvon and Wadsworth (1997). Briefly, cells were fixed at room temperature for 1 min with 1 ml of 4% formaldehyde and 0.25% glutaraldehyde in PBS containing 0.1% Tween-20 (PBST). The coverslips were then further fixed with 1 ml of 4% formaldehyde and 0.25% glutaraldehyde in PHEM lysis buffer (0.5% Triton X-100, 1 mM MgSO4, 5 mM ethylene glycol tetraacetic acid, and 80 mM 1,4-piperazinediethanesulfonic acid, pH 6.8) for 6 min. Next 1 ml of 50 mM NH4Cl was added to each coverslip for 2 min to quench the reaction, and the cells were washed three times with PBST. Samples were stained with 1:10 anti-tubulin antibody (mouse monoclonal 12G10 anti–α- or E7 anti–β-tubulin; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) in PBST for 30 min, washed once in PBST, and then incubated in 200 μl of 1:200 Alexa 488 goat anti-mouse antibody (Jackson ImmunoResearch) in PBST for 30 min. Samples were imaged using the Nikon A1R laser scanning confocal microscope to visualize the state of the microtubule cytoskeleton.
Detection of actin localization when microtubules are depolymerized
Cells were plated overnight in a WillCo or ibidi imaging dish and then treated with 800 nM nocodazole for 45 min in CO2-independent medium. Cells were fixed at room temperature with 1 ml of 4% formaldehyde and 0.25% gluteraldehyde in PBS containing 0.1% Triton X-100 and 0.1 μg/ml rhodamine phalloidin for 6 min and then imaged with a Nikon A1R laser scanning confocal microscope. To measure the increase in cortical fluorescence intensity in the presence or absence of nocodazole, the ImageJ Threshold tool was used to set maximum and minimum threshold levels so that only the phalloidin label at the cortex was highlighted and the mean fluorescence intensity of this region was measured.
Cell death assay
Cells were plated as previously described and treated with 800 nM nocodazole, 500 nM latrunculin, 500 nM cytochalasin, 20 μM LY294002, or 20 nM wortmannin in CO2-independent medium for 45 min before particle exposure. Cells were then exposed to 15 μg/cm2 uncoated silica particles or 40 μl of latex beads in medium containing 0.1 μg/ml propidium iodide and imaged on a wide-field microscope after 0, 4, and 10 h. Approximately 300 cells, three fields of view from each time point, were scored manually for nuclear fluorescence arising from cell death.
Supplementary Material
Acknowledgments
We thank Gaurav Joshi for providing the MH-S macrophage cells that stably express GFP-actin, Sergio Grinstein at the Hospital for Sick Children (Toronto, Canada) for providing the AKT-PH-GFP and 2xFYVE-GFP constructs, and Roland Wedlich-Soldner at the Max Planck Institute of Biochemistry (Munich, Germany) for the mRFP-Ruby Lifeact construct. Carol Norris at the University of Connecticut Confocal Microscopy Facility provided microscopy assistance, and we thank Sergio Grinstein, Michael Lynes, Adam Zweifach, Andrea Hubbard, and Akiko Nishiyama for many informative discussions regarding this research.
Abbreviations used:
- Ab
antibody
- COZ
complement-opsonized zymosan
- GFP
green fluorescent protein
- PIP
phosphatidylinositol phosphate
- PMA
phorbol myristate acetate.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-08-1301) on November 26, 2014.
*Present address: Center for Academic Programs, Unit 4170, University of Connecticut, 368 Fairfield Way, Storrs, CT 06269.
REFERENCES
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Allen LA, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajno L, Peng XR, Schreiber AD, Moore HP, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149:697–706. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini F, Gundersen G. Formins and microtubules. Biochim Biophys Acta. 2010;1803:164–173. doi: 10.1016/j.bbamcr.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer CA, Holian A. Scavenger receptor class A type I/II (CD204) null mice fail to develop fibrosis following silica exposure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L186–195. doi: 10.1152/ajplung.00474.2004. [DOI] [PubMed] [Google Scholar]
- Blackford JA, Jr, Antonini JM, Castranova V, Dey RD. Intratracheal instillation of silica up-regulates inducible nitric oxide synthase gene expression and increases nitric oxide production in alveolar macrophages and neutrophils. Am J Respir Cell Mol Biol. 1994;11:426–431. doi: 10.1165/ajrcmb.11.4.7522485. [DOI] [PubMed] [Google Scholar]
- Bohdanowicz M, Cosio G, Backer JM, Grinstein S. Class I and class III phosphoinositide 3-kinases are required for actin polymerization that propels phagosomes. J Cell Biol. 2010;191:999–1012. doi: 10.1083/jcb.201004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Caron E, Self AJ, Hall A. The GTPase Rap1 controls functional activation of macrophage integrin alphaMbeta2 by LPS and other inflammatory mediators. Curr Biol. 2000;10:974–978. doi: 10.1016/s0960-9822(00)00641-2. [DOI] [PubMed] [Google Scholar]
- Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- Caumont AS, Galas MC, Vitale N, Aunis D, Bader MF. Regulated exocytosis in chromaffin cells. Translocation of ARF6 stimulates a plasma membrane-associated phospholipase D. J Biol Chem. 1998;273:1373–1379. doi: 10.1074/jbc.273.3.1373. [DOI] [PubMed] [Google Scholar]
- Chao SK, Hamilton RF, Pfau JC, Holian A. Cell surface regulation of silica-induced apoptosis by the SR-A scavenger receptor in a murine lung macrophage cell line (MH-S) Toxicol Appl Pharmacol. 2001;174:10–16. doi: 10.1006/taap.2001.9190. [DOI] [PubMed] [Google Scholar]
- Costantini LM, Gilberti RM, Knecht DA. The phagocytosis and toxicity of amorphous silica. PLoS One. 2011;6:e14647. doi: 10.1371/journal.pone.0014647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J Biol Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–234. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Gupton SL, Gertler FB. The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a001800. a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins M. Biogenesis of phagolysosomes: the “kiss and run” hypothesis. Trends Cell Biol. 1995;5:183–186. doi: 10.1016/s0962-8924(00)88989-8. [DOI] [PubMed] [Google Scholar]
- Duclos S, Corsini R, Desjardins M. Remodeling of endosomes during lysosome biogenesis involves “kiss and run” fusion events regulated by rab5. J Cell Sci. 2003;116:907–918. doi: 10.1242/jcs.00259. [DOI] [PubMed] [Google Scholar]
- Fitzer-Attas CJ, Lowry M, Crowley MT, Finn AJ, Meng F, DeFranco AL, Lowell CA. Fcgamma receptor-mediated phagocytosis in macrophages lacking the Src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191:669–682. doi: 10.1084/jem.191.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubini B. Surface chemistry and quartz hazard. Ann Occup Hyg. 1998;42:521–530. doi: 10.1016/s0003-4878(98)00066-0. [DOI] [PubMed] [Google Scholar]
- Ghazizadeh S, Bolen JB, Fleit HB. Physical and functional association of Src-related protein tyrosine kinases with Fc gamma RII in monocytic THP-1 cells. J Biol Chem. 1994;269:8878–8884. [PubMed] [Google Scholar]
- Gilberti RM, Joshi GN, Knecht DA. The phagocytosis of crystalline silica particles by macrophages. Am J Respir Cell Mol Biol. 2008;39:619–627. doi: 10.1165/rcmb.2008-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. The macrophage. Bioessays. 1995;17:977–986. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- Greenberg S, Grinstein S. Phagocytosis and innate immunity. Curr Opin Immunol. 2002;14:136–145. doi: 10.1016/s0952-7915(01)00309-0. [DOI] [PubMed] [Google Scholar]
- Griffin FM, Jr, Griffin JA, Silverstein SC. Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J Exp Med. 1976;144:788–809. doi: 10.1084/jem.144.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Botelho RJ, Yu M, Grinstein S, Neel BG. Critical role for scaffolding adapter Gab2 in Fc gamma R-mediated phagocytosis. J Cell Biol. 2003;161:1151–1161. doi: 10.1083/jcb.200212158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl P, Duffin R, Kramer U, Hohr D, Schins RP, Borm PJ, Albrecht C. Actin plays a crucial role in the phagocytosis and biological response to respirable quartz particles in macrophages. Arch Toxicol. 2007;81:459–470. doi: 10.1007/s00204-007-0178-5. [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Schreiber A, Zhang W, Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcgamma receptors in macrophages. J Exp Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AB, Gakidis MA, Glogauer M, Wilsbacher JL, Gao S, Swat W, Brugge JS. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcgammaR- and complement-mediated phagocytosis. Immunity. 2006;24:305–316. doi: 10.1016/j.immuni.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Hamilton R, de Villiers WJS, Holian A. Class A type II scavenger receptor mediates silica-induced apoptosis in Chinese hamster ovary cell line. Toxicol Appl Pharmacol. 2000;162:100–106. doi: 10.1006/taap.1999.8799. [DOI] [PubMed] [Google Scholar]
- Hamilton RF, Jr, Thakur SA, Mayfair JK, Holian A. MARCO mediates silica uptake and toxicity in alveolar macrophages from C57BL/6 mice. J Biol Chem. 2006:34218–34226. doi: 10.1074/jbc.M605229200. [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Watanabe H, Kawamoto K, Nakayama K, Morris AJ, Frohman MA, Kanaho Y. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Huaux F. New developments in the understanding of immunology in silicosis. Curr Opin Allergy Clin Immunol. 2007;7:168–173. doi: 10.1097/ACI.0b013e32802bf8a5. [DOI] [PubMed] [Google Scholar]
- Indik Z, Kelly C, Chien P, Levinson AI, Schreiber AD. Human Fc gamma RII, in the absence of other Fc gamma receptors, mediates a phagocytic signal. J Clin Invest. 1991;88:1766–1771. doi: 10.1172/JCI115496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Hamilton RF, Li L, Holian A. Silica-induced apoptosis mediated via scavenger receptor in human alveolar macrophages. Toxicol Appl Pharmacol. 1996;141:84–92. doi: 10.1006/taap.1996.0263. [DOI] [PubMed] [Google Scholar]
- Iyer R, Holian A. Involvement of the ICE family of proteases in silica-induced apoptosis in human alveolar macrophages. Am J Physiol. 1997;273:L760–L767. doi: 10.1152/ajplung.1997.273.4.L760. [DOI] [PubMed] [Google Scholar]
- Joshi GN, Knecht DA. Silica phagocytosis causes apoptosis and necrosis by different temporal and molecular pathways in alveolar macrophages. Apoptosis. 2013;18:271–285. doi: 10.1007/s10495-012-0798-y. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Brumell J, Al-Alawi N, Latour S, Cheng A, Veillette A, Grinstein S, Pawson T. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol Cell Biol. 1998;18:4209–4220. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobzik L. Lung macrophage uptake of unopsonized environmental particulates. Role of scavenger-type receptors. J Immunol. 1995;155:367–376. [PubMed] [Google Scholar]
- Lee CY, Herant M, Heinrich V. Target-specific mechanics of phagocytosis: protrusive neutrophil response to zymosan differs from the uptake of antibody-tagged pathogens. J Cell Sci. 2011;124:1106–1114. doi: 10.1242/jcs.078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz E, Herit F, Le Clainche C, Bourdoncle P, Perez F, Niedergang F. The microtubule-binding protein CLIP-170 coordinates mDia1 and actin reorganization during CR3-mediated phagocytosis. J Cell Biol. 2008;183:1287–1298. doi: 10.1083/jcb.200807023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loijens JC, Boronenkov IV, Parker GJ, Anderson RA. The phosphatidylinositol 4-phosphate 5-kinase family. Adv Enzyme Regul. 1996;36:115–140. doi: 10.1016/0065-2571(95)00005-4. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Insall RH. Signaling to actin dynamics. J Cell Biol. 1999;146:267–272. doi: 10.1083/jcb.146.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JG, Booth JW, Stambolic V, Mak T, Balla T, Schreiber AD, Meyer T, Grinstein S. Restricted accumulation of phosphatidylinositol 3-kinase products in a plasmalemmal subdomain during Fc gamma receptor-mediated phagocytosis. J Cell Biol. 2001;153:1369–1380. doi: 10.1083/jcb.153.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May RC, Caron E, Hall A, Machesky LM. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat Cell Biol. 2000;2:246–248. doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- May RC, Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci. 2001;114:1061–1077. doi: 10.1242/jcs.114.6.1061. [DOI] [PubMed] [Google Scholar]
- Newman SL, Mikus LK, Tucci MA. Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J Immunol. 1991;146:967–974. [PubMed] [Google Scholar]
- Palecanda A, Kobzik L. Receptors for unopsonized particles: the role of alveolar macrophage scavenger receptors. Curr Mol Med. 2001;1:589–595. doi: 10.2174/1566524013363384. [DOI] [PubMed] [Google Scholar]
- Patel PC, Harrison RE. Membrane ruffles capture C3bi-opsonized particles in activated macrophages. Mol Biol Cell. 2008;19:4628–4639. doi: 10.1091/mbc.E08-02-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt A, Mayorga LS, Stahl PD, Schwartz AL. Alterations in the protein composition of maturing phagosomes. J Clin Invest. 1992;90:1978–1983. doi: 10.1172/JCI116077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607. doi: 10.1038/nmeth.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott CC, Botelho RJ, Grinstein S. Phagosome maturation: a few bugs in the system. J Membr Biol. 2003;193:137–152. doi: 10.1007/s00232-002-2008-2. [DOI] [PubMed] [Google Scholar]
- Scott CC, Dobson W, Botelho RJ, Coady-Osberg N, Chavrier P, Knecht DA, Heath C, Stahl P, Grinstein S. Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J Cell Biol. 2005;169:139–149. doi: 10.1083/jcb.200412162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HM, Zhang Z, Zhang QF, Ong CN. Reactive oxygen species and caspase activation mediate silica-induced apoptosis in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2001;280:L10–L17. doi: 10.1152/ajplung.2001.280.1.L10. [DOI] [PubMed] [Google Scholar]
- Shi Y, Tohyama Y, Kadono T, He J, Miah SM, Hazama R, Tanaka C, Tohyama K, Yamamura H. Protein-tyrosine kinase Syk is required for pathogen engulfment in complement-mediated phagocytosis. Blood. 2006;107:4554–4562. doi: 10.1182/blood-2005-09-3616. [DOI] [PubMed] [Google Scholar]
- Stenmark H, Gillooly DJ. Intracellular trafficking and turnover of phosphatidylinositol 3-phosphate. Semin Cell Dev Biol. 2001;12:193–199. doi: 10.1006/scdb.2000.0236. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kono H, Hirose N, Okada M, Yamamoto T, Yamamoto K, Honda Z. Differential involvement of Src family kinases in Fc gamma receptor-mediated phagocytosis. J Immunol. 2000;165:473–482. doi: 10.4049/jimmunol.165.1.473. [DOI] [PubMed] [Google Scholar]
- Swanson JA, Baer SC. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5:89–93. doi: 10.1016/s0962-8924(00)88956-4. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- Thakur SA, Beamer CA, Migliaccio CT, Holian A. Critical role of MARCO in crystalline silica-induced pulmonary inflammation. Toxicol Sci. 2009;108:462–471. doi: 10.1093/toxsci/kfp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau M, Giardina C, Hubbard AK. Silica-induced caspase activation in mouse alveolar macrophages is dependent upon mitochondrial integrity and aspartic proteolysis. Toxicol Sci. 2003;76:91–101. doi: 10.1093/toxsci/kfg178. [DOI] [PubMed] [Google Scholar]
- Thibodeau MS, Giardina C, Knecht DA, Helble J, Hubbard AK. Silica-induced apoptosis in mouse alveolar macrophages is initiated by lysosomal enzyme activity. Toxicol Sci. 2004;80:34–48. doi: 10.1093/toxsci/kfh121. [DOI] [PubMed] [Google Scholar]
- Tjelle TE, Lovdal T, Berg T. Phagosome dynamics and function. Bioessays. 2000;22:255–263. doi: 10.1002/(SICI)1521-1878(200003)22:3<255::AID-BIES7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- Vallyathan V, Shi X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ Health Perspect. 1997;105((Suppl 1)):165–177. doi: 10.1289/ehp.97105s1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Grinstein S. Phagosome maturation: aging gracefully. Biochem J. 2002;366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Rameh L, Brachmann SM, Matsuo T, Davidson HW, Schreiber A, Backer JM, Cantley LC, Grinstein S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J Cell Biol. 2001;155:19–25. doi: 10.1083/jcb.200107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, Schreiber AD, Stahl PD, Grinstein S. Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol. 2003;23:2501–2514. doi: 10.1128/MCB.23.7.2501-2514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung T, Ozdamar B, Paroutis P, Grinstein S. Lipid metabolism and dynamics during phagocytosis. Curr Opin Cell Biol. 2006;18:429–437. doi: 10.1016/j.ceb.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Yvon AM, Wadsworth P. Non-centrosomal microtubule formation and measurement of minus end microtubule dynamics in A498 cells. J Cell Sci. 1997;110:2391–2401. doi: 10.1242/jcs.110.19.2391. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Kee YS, Poirier CC, Jelinek C, Osborne J, Divi S, Surcel A, Will ME, Eggert US, Muller-Taubenberger A, et al. 14–3-3 coordinates microtubules, Rac, and myosin II to control cell mechanics and cytokinesis. Curr Biol. 2010a;20:1881–1889. doi: 10.1016/j.cub.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Kim HY, Wang JH, Davidson LA. Macroscopic stiffening of embryonic tissues via microtubules, RhoGEF and the assembly of contractile bundles of actomyosin. Development. 2010b;137:2785–2794. doi: 10.1242/dev.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.