The levels of expression, distribution, and association of all of the VAMPs expressed in 3T3-L1 adipocytes are characterized. This is the first systematic analysis of all members of this protein family for any cell type.
Abstract
The fusion of GLUT4-containing vesicles with the plasma membrane of adipocytes is a key facet of insulin action. This process is mediated by the formation of functional soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) complexes between the plasma membrane t-SNARE complex and the vesicle v-SNARE or VAMP. The t-SNARE complex consists of Syntaxin4 and SNAP23, and whereas many studies identify VAMP2 as the v-SNARE, others suggest that either VAMP3 or VAMP8 may also fulfil this role. Here we characterized the levels of expression, distribution, and association of all the VAMPs expressed in 3T3-L1 adipocytes to provide the first systematic analysis of all members of this protein family for any cell type. Despite our finding that all VAMP isoforms form SDS-resistant SNARE complexes with Syntaxin4/SNAP23 in vitro, a combination of levels of expression (which vary by >30-fold), subcellular distribution, and coimmunoprecipitation analyses lead us to propose that VAMP2 is the major v-SNARE involved in GLUT4 trafficking to the surface of 3T3-L1 adipocytes.
INTRODUCTION
Insulin stimulates glucose transport in adipose and muscle tissue by inducing the movement (“translocation”) of intracellular vesicles containing the glucose transporter, GLUT4, to the cell surface (Leto and Saltiel, 2012). Once at the plasma membrane, these vesicles dock and fuse, resulting in increased levels of functional glucose transporters at the cell surface and thus elevated levels of glucose transport into the cell (Bryant et al., 2002). This translocation mechanism appears to be defective in individuals with type 2 diabetes or insulin resistance, and thus much effort has been focused on defining the molecular basis of this key action of insulin (Rizza and Butler, 1990; Shepherd and Kahn, 1999).
In the absence of insulin, GLUT4 is effectively sequestered inside the cell. This is achieved via two interrelated endosomal trafficking cycles that culminate in a significant fraction of GLUT4 populating specialized GLUT4-storage vesicles (GSVs; Bryant et al., 2002). Defining how these GSVs dock and fuse with the cell surface has focused attention on the soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) machinery for membrane fusion, as SNARE proteins are involved in all membrane-trafficking steps in eukaryotes. There is agreement that the plasma membrane–localized target (t)-SNARE complex comprises Syntaxin4 and SNAP23, but the identity of the vesicle (v)-SNARE involved in GSV fusion with the cell surface has been more controversial (Cheatham et al., 1996; Zhao et al., 2009; Bryant and Gould, 2011). Multiple v-SNAREs have been identified in GSVs, including vesicle-associated membrane protein 2 (VAMP2), VAMP3, and VAMP8 (Volchuk et al., 1995; Larance et al., 2005). These v-SNAREs have all been shown to play a role in exocytosis in other cell types and in each case do so via interaction with Syntaxin4/SNAP23 (Bajno et al., 2000; Schoch et al., 2001; Polgar et al., 2002; Cosen-Binker et al., 2008). The majority of studies of GSV exocytosis in adipocytes have focused on VAMP2, and studies using small interfering RNA (siRNA; Williams and Pessin, 2008; Kawaguchi et al., 2010), dominant-negative inhibitory fragment expression (Millar et al., 1999), or peptide interference (Martin et al., 1998) have all supported a role for VAMP2 in insulin-stimulated glucose transport/GLUT4 translocation. However, data using toxin-specific cleavage of VAMP2 have been more controversial, with some studies reporting significant inhibition of insulin-stimulated Glut4 translocation (Randhawa et al., 2000) and others not (Hajduch et al., 1997).
A study using knockout mice and toxin-based approaches to interrogate VAMP function demonstrated that simultaneous disruption of VAMPs 2, 3, and 8 was required to completely block insulin-stimulated GLUT4 insertion into the plasma membrane of adipocytes; this defect could be rescued by reexpression of VAMP2, VAMP3, or VAMP8 alone, a result interpreted to imply plasticity in the requirement for v-SNARE proteins in GLUT4 trafficking to the plasma membrane (Zhao et al., 2009). This study appears contradictory to others, in which disruption of VAMP2 function alone was sufficient to impair GLUT4 translocation (Martin et al., 1998; Millar et al., 1999; Williams and Pessin, 2008; Kawaguchi et al., 2010; Bryant and Gould, 2011). There are several possible explanations for these apparent discrepancies; key among these is that the absolute levels of the different VAMP isoforms when reexpressed in adipocytes are not known. This is important, as GLUT4 populates multiple intracellular compartments, and this depletion or inhibition of one arm of GLUT4 traffic (e.g., by siRNA or toxin treatment) may drive GLUT4 along another route to the cell surface. Hence systematic analysis of levels of expression, function, and distribution and the effect of insulin is warranted. Here we report such an analysis.
We show that all VAMP isoforms are capable of forming an SDS-resistant SNARE complex with Syntaxin4/SNAP23 in vitro. However, we found that VAMP isoforms differ widely in their levels of expression in 3T3-L1 adipocytes. VAMP3 and VAMP8 are expressed approximately twofold and approximately fourfold higher than VAMP2, respectively, but unlike VAMPs 2 and 3, VAMP8 levels do not increase upon differentiation of 3T3-L1 fibroblasts to adipocytes (a key hallmark of the GLUT4 trafficking machinery; El-Jack et al., 1999). VAMP2 and VAMP3 both exhibit insulin-stimulated translocation to the cell surface, whereas VAMP8 does not. We also show that VAMP2 is localized within a GSV-enriched fraction to a far greater degree than VAMP3. Such data support the notion that VAMP2 is the major v-SNARE involved in GLUT4 trafficking from GSVs to the plasma membrane. Consistent with this, we show that immunoprecipitation of Syntaxin4 coprecipitates VAMP2 and VAMP5 but none of the other VAMP isoforms. Our data therefore support the notion that VAMP2 is the major v-SNARE involved in GLUT4 trafficking to the surface of 3T3-L1 adipocytes.
RESULTS
All VAMP isoforms can form SDS-resistant SNARE complexes with Syntaxin4/SNAP23
A crucial facet of the debate regarding which VAMP isoform(s) might be key for insulin-regulated GLUT4 delivery to the cell surface is whether these different VAMP isoforms form SNARE complexes with Syntaxin4 and SNAP23. Although this has been shown for some isoforms, (specifically VAMPs 2, 3, and 8; Polgar et al., 2002), it is not clear how specific this is. To address this, we took advantage of the ability of SNAREs to form SDS-resistant ternary complexes. As shown in Figure 1, all of the VAMPs were capable of forming a ternary complex, consistent with other studies of Syntaxin1A, confirming that the interactions between t-SNAREs and v-SNAREs in vitro are promiscuous (Yang et al., 1999). Such data are consistent with the notion that any of the VAMPs could, in principle, mediate fusion with the plasma membrane.
FIGURE 1:
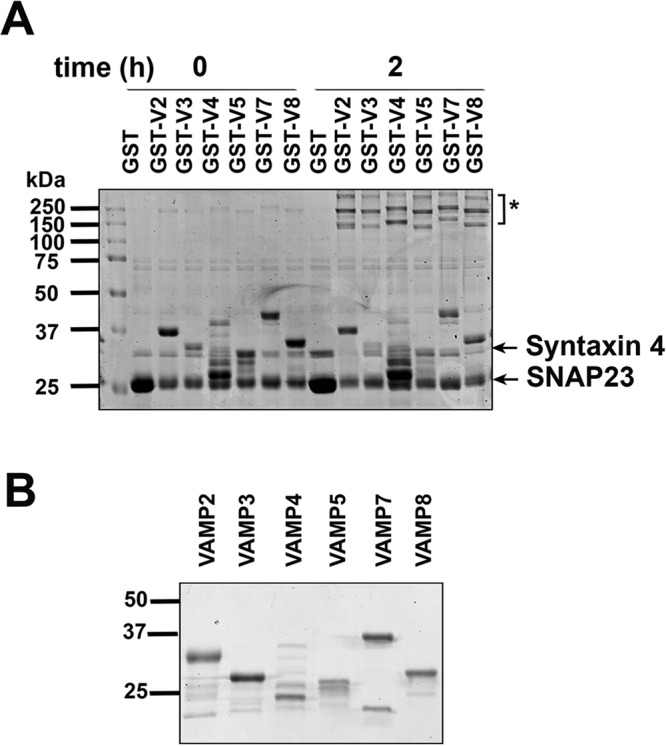
All VAMP isoforms form SDS-resistant complexes with Syntaxin4 and SNAP23. (A) Equimolar (∼4 μM) amounts of His-SNAP23, Syntaxin4, and GST-tagged VAMP (or GST alone) were combined, immediately mixed with 5× LSB, and heated to 95°C or incubated for 2 h at 4°C with rotation before being mixed with 5× LSB and heated to 95°C. Samples were subjected to SDS–PAGE, followed by staining with Coomassie brilliant blue. Data from a typical experiment repeated three times; molecular marker sizes are shown in kilodaltons. Molecular weights: His-SNAP23, ∼25 kDa; Syntaxin4, ∼32 kDa. The SNARE complex runs >110 kDa and is indicated by an asterisk. (B) Input VAMP isoforms used in the assay in A. A 1- mg amount of each purified recombinant VAMP isoform was subjected to SDS–PAGE, and protein was stained with Coomassie brilliant blue; molecular marker sizes are shown in kilodaltons.
VAMPs are selectively up-regulated during differentiation
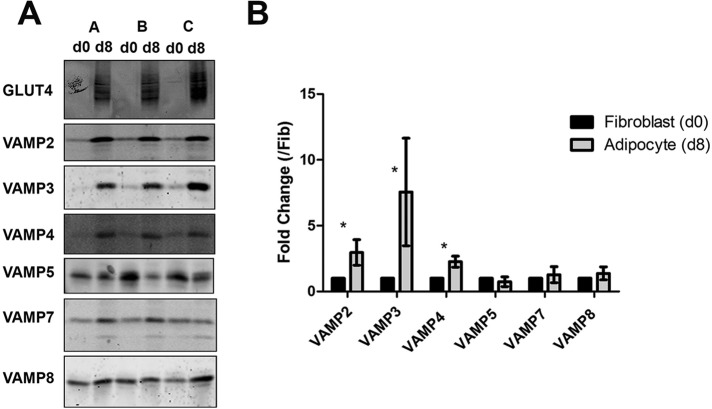
Many studies have shown that key regulatory molecules involved in insulin-stimulated GLUT4 trafficking are up-regulated upon differentiation of 3T3-L1 fibroblasts into adipocytes; these include GLUT4, VAMP2, Syntaxin16, and insulin-responsive aminopeptidase (El-Jack et al., 1999; Perera et al., 2003; Shewan et al., 2003). We examined whether the levels of VAMP isoforms exhibited similar increases. Figure 2 shows an analysis of three different batches of 3T3-L1 fibroblasts and adipocytes immunoblotted for the different VAMPs. In each case, total cell numbers were estimated by counting propidium iodide–stained nuclei, and each lane on the blot shows membranes from an equivalent number of cells. Levels of VAMPs 2–4 all increased significantly during differentiation, but levels of VAMPs 5, 7, and 8 did not. Of note, under the conditions used for immunoblotting, the antibodies used were specific for the isoform indicated (unpublished data).
FIGURE 2:
Selective VAMP isoforms are up-regulated during differentiation of 3T3-L1 adipocytes. (A) Equal numbers of fibroblast and adipocyte cells, quantified using propidium iodide staining of nuclei, were homogenized and subjected to centrifugation at 95,000 × g for 1 h to pellet to the total membranes as described. Samples representing equal numbers of cells from day 0 (fibroblasts; d0) or day 8 (adipocytes; d8) were then subjected to SDS–PAGE, followed by immunoblotting with anti-GLUT4 and anti-VAMP antibodies as indicated. Three batches of membranes are displayed on each gel (A–C); d0, fibroblast. (B) The changes in VAMP levels upon differentiation were quantified. Values represent the means of three separate experiments in which fold changes compared with fibroblasts are shown for each VAMP member; values were compared using Student's t test, *p < 0.05.
It is important to determine the absolute levels of expression of each of the VAMP isoforms, and we used quantitative immunoblotting with recombinant protein standards to achieve this (Hickson et al., 2000). Total membrane fractions were prepared as described, and known amounts of membrane protein were separated on SDS–PAGE gels alongside known amounts of recombinant VAMP. Comparison of the immunoblot signals from the total membrane sample can thus be compared with known standards to allow estimation of the total amount of VAMP-x present in the membrane sample studied and then from a given number of cells, as the latter was determined in parallel using propidium iodide staining of nuclei. In our hands, a 10-cm dish of 3T3-L1 adipocytes contained 107 cells and gave ∼2.5 mg of protein (see legend to Figure 3). This relies on the antibodies in question cross-reacting with endogenous VAMPs to the same extent as is observed for purified recombinant proteins; although we have no reason to believe that this is not the case, the data should be interpreted with this caveat in mind. Representative examples of such analysis are shown in Figure 3, with the data for all VAMPs shown in Figure 3B. These data reveal wide differences in the levels of expression of the different VAMPs, with VAMPs 5 and 7 expressed at very low levels (<1 × 105 copies/cell). VAMP4 and VAMP2 were expressed at moderate levels (4.8 × 105 and 8.6 × 105 copies/cell, respectively). By contrast, VAMP3 and VAMP8 were highly expressed (1.5 × 106 and 3.5 × 106 copies/cell, respectively).
FIGURE 3:
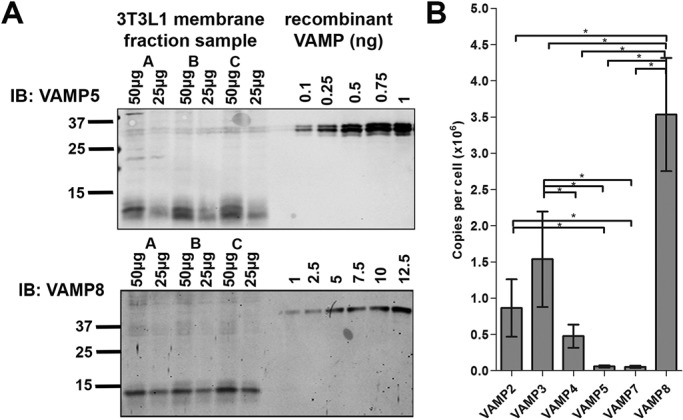
Quantification of VAMP levels using quantitative immunoblotting. Total membrane fractions from day 8 adipocytes were analyzed by immunoblotting alongside known amounts of purified recombinant VAMP (Figure 1B). Two examples, VAMP3 and VAMP8; each immunoblot contains membrane fractions (25 and 50 μg) from three separate batches of cells (batches A–C). Data from a typical experiment. Depending on the VAMP being analyzed, a range of different protein loads was always used on multiple blots to ensure that detection was on the linear range of the response. (B) Quantification of these experiments. In our hands, one 10-cm dish contained 107 cells and 2.5 mg of membrane protein (Biber and Lienhard, 1986; Hickson et al., 2000). For each batch of cells, we assayed the total membrane protein recovered and related this to the number of cells quantified using propidium iodide staining as outlined in the legend to Figure 2. The data shown are the mean and SD values obtained from three separate batches of 3T3-L1 adipocyte membranes; asterisk indicates a significant change, p < 0.05.
VAMP isoforms are differentially distributed
The data just described are important for comparing total cellular amounts, but they do not provide insight into the potential local concentration of VAMP isoforms among different cellular compartments; this is important, as such local concentrations could be significantly higher. With this in mind, we examined the distribution of the different VAMP isoforms among subcellular fractions comprising plasma membranes (PMs), light density membranes (LDMs; the location of the majority of GLUT4), and heavy membrane fractions (HDMs; Supplemental Figure S1 quantifies marker protein expression in these fractions to verify the effectiveness of the fractionation). In the absence of insulin, both VAMP2 and VAMP3 were predominantly (>60%) localized to the LDM fraction, consistent with previous studies of these VAMPs. By contrast, the remaining VAMPs exhibited broadly similar levels in both PM and LDM fractions (Figure 4A). Striking differences were observed when the effects of insulin on distribution were examined (quantified in Figure 4B). Consistent with previous work (Cheatham et al., 1996), insulin induced redistribution of VAMP2 and VAMP3 to the plasma membrane. Plasma membrane levels of the other VAMP isoforms did not increase in response to insulin (note that a trend toward increased levels of VAMP5 is evident in these data, but low levels of expression made this difficult to quantify accurately). Decreased levels in the LDM were observed for VAMP8, but increases in PM or HDM fractions did not reach statistical significance.
FIGURE 4:
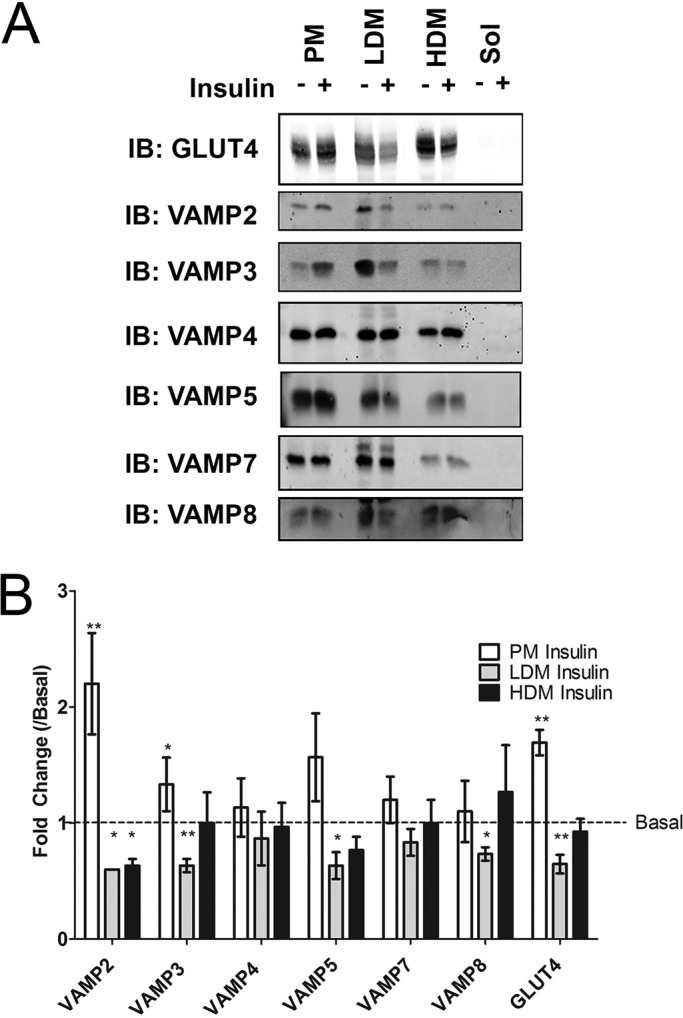
Subcellular distribution of VAMP isoforms in basal and insulin-treated adipocytes. 3T3 L1 adipocytes were treated with (+) or without (–) 1 μM insulin for 20 min. Subcellular fractionation was performed as described generating PM, LDM, HDM, and soluble fractions (Sol; see also Supplemental Figure S1). Fractions were resuspended in equal volumes of 1× LSB and subjected to SDS–PAGE and immunoblotting with the indicated antibodies. (A) Representative immunoblots, in each case repeated with a minimum of three separate platings of cells. (B) Quantification of the changes in the distribution of each VAMP isoform in each fraction in response to insulin expressed relative to the basal levels in each fraction. Values represent means ± SD of three separate experiments; fold change compared with basal values were compared using Student's t test, *p < 0.05, **p < 0.01.
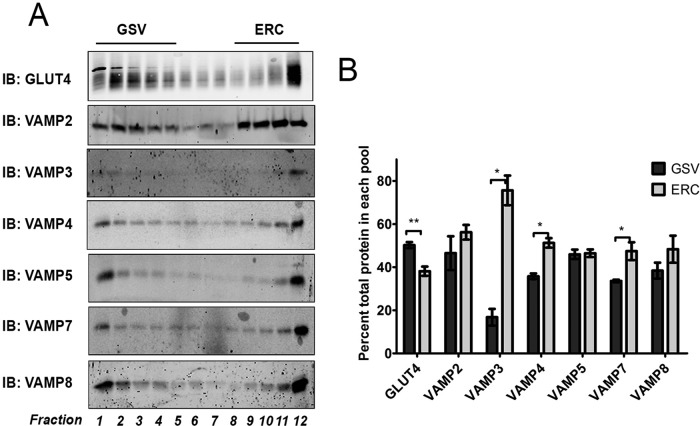
GLUT4 within the LDM fraction of adipocytes is known to be present in both early/recycling endosomes and GSVs. Iodixanol gradient analysis been used to resolve these fractions (Hashiramoto and James, 2000), allowing comparison of the VAMP distribution among these fractions to be determined (Figure 5A). Consistent with previous work, we find that VAMP2 is present within both fractions approximately equally (Martin et al., 1996; Hashiramoto and James, 2000; Maier and Gould, 2000); similar distributions were observed for VAMP5 and VAMP8. VAMP3 is predominantly localized to the endosomal/recycling fractions (>75% in the endocytic recycling compartment [ERC]; Figure 5B), also consistent with previous studies (Volchuk et al., 1995; Martin et al., 1998). Similarly, VAMP4 and VAMP7 are found at somewhat greater levels in the ERC fractions.
FIGURE 5:
VAMP isoforms are differentially distributed on iodixanol gradients. (A) LDM fractions from serum-starved (basal) 3T3-L1 adipocytes were resuspended in HES buffer (250 mM sucrose, 20 mM HEPES, pH 7.4, 1 mM EDTA) and centrifuged on self-forming iodixanol gradients as described. Twelve equal-volume fractions were collected and subjected to SDS–PAGE, followed by immunoblotting with the indicated antibodies. Fractions were loaded in order of decreasing density; fractions 1–5 represent GSV-enriched fractions, and 9–12 represent ERCs (Hashiramoto and James, 2000). (A) Immunoblots from a typical experiment and (B) the mean values ± SD for percentage of total protein across all fractions found in the GSV and ERC pools from three separate experiments; values were compared using Student's t test, *p < 0.05, **p < 0.01.
Syntaxin4 coimmunoprecipitates VAMP2
To examine interactions of the VAMP family members with Syntaxin4 in vivo, we immunoprecipitated Syntaxin4 from basal and insulin-stimulated adipocytes and probed for coimmunoprecipitation of VAMP isoforms. We observed Syntaxin4/VAMP2 coimmunoprecipitation in insulin-stimulated cells and Syntaxin4/VAMP5 interactions in basal and insulin-stimulated conditions (the latter is consistent with data from cardiomyocytes; Schwenk et al., 2010) but could detect no other VAMP isoform in Syntaxin4 immunoprecipitates under either condition (Figure 6). By contrast, VAMP4 was readily coimmunoprecipitated by anti-Syntaxin16 (Sadler et al., 2015). Of interest, we were unable to demonstrate coimmunoprecipitation of Syntaxin4 with VAMP3 in response to insulin at times of exposure of insulin ranging from 3 to 60 min (unpublished data), suggesting that the delivery of GLUT4 to the cell surface uses the same SNARE machinery at all time points.
FIGURE 6:
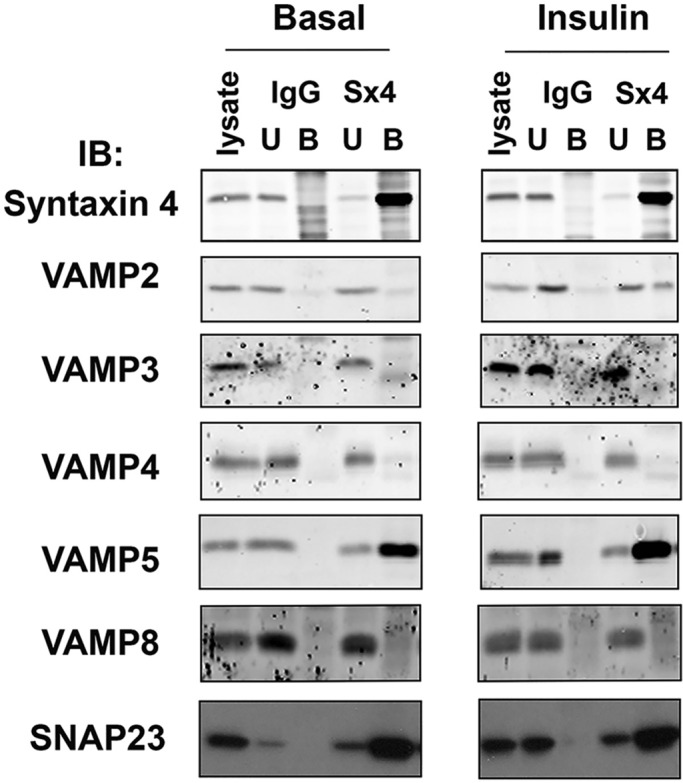
VAMP2 coimmunoprecipitates with Syntaxin4 in adipocytes. A 5 μl amount of anti-Syntaxin4 (Sx4) or random rabbit immunoglobulin G (IgG) was used to immunoprecipitate from 1.5 mg of lysate prepared from 3T3-L1 adipocytes incubated without (Basal) or with 1 μM insulin (Insulin) for 30 min as described. Immunoprecipitated proteins were recovered by centrifugation, washed, and eluted in 2× LSB. Samples were subjected to SDS–PAGE and immunoblotting with the indicated antibodies. Data from a typical experiment, repeated at least three times with qualitatively similar results; for each immunoprecipitation, the basal and insulin-stimulated samples were run on the same gel with other samples, which have been omitted here for clarity. Lysate, the starting cell lysate; U, material remaining in the lysate after the immunoprecipitation; B, the immunoprecipitated material.
DISCUSSION
Studies have reported that siRNA-mediated depletion of VAMP2 results in abrogated insulin-stimulated GLUT4 translocation to the plasma membrane of adipocytes, L6 muscle cells, and HL-1 cardiomyocytes, and VAMP5 was also shown to mediate insulin-stimulated GLUT4 translocation (Randhawa et al., 2004; Williams and Pessin, 2008; Kawaguchi et al., 2010; Schwenk et al., 2010). Similarly, studies using either inhibitory peptides or expression of SNARE protein domains have supported a role for VAMP2 in insulin-stimulated GLUT4 translocation, with VAMP3 mediating translocation by other stimuli, such as osmotic shock, GTPγS, or valinomycin; VAMP7 has also been reported to mediate GLUT4 translocation in response to osmotic shock (Millar et al., 1999; Williams and Pessin, 2008).
Paradoxically, other studies have suggested that many SNARE-mediated trafficking events use multiple v-SNAREs (e.g., Liu and Barlowe, 2002), suggesting functional redundancy at the level of VAMPs (Yang et al., 1999). In support of this model, Zhao et al. (2009) showed that VAMP2, 3, or 8 could each separately restore insulin-stimulated GLUT4 translocation in cells devoid of endogenous VAMP2, 3, or 8. Such data led these authors to propose that these VAMPs could operate as v-SNAREs for GLUT4 translocation interchangeably. Consistent with this notion, we show here that all of the VAMPs expressed in 3T3-L1 adipocytes are capable of forming an SDS-resistant SNARE complex with Syntaxin4 and SNAP23. It should be noted, however, that others have reported that VAMP3 cannot form a fusogenic pairing with Sx4/SNAP23 but does so with Sx4/SNAP25 (Hu et al., 2007). Studies in which one or more VAMP isoform is absent (through either genetic or siRNA-mediated means) raise the possibility that other trafficking routes can compensate and deliver GLUT4 to the cell surface by a distinct route. A prerequisite of this model is that VAMPs are sorted into distinct compartments. Zhao et al. (2009) argued this is not the case, based on colocalization of different VAMPs and GLUT4 in isolated membrane fractions stained with anti-VAMP antibodies, as between 20 and 50% overlap was observed between VAMP2, VAMP3, VAMP8, and GLUT4. One problem with this approach is that a vesicle with quite different levels of VAMP-x and VAMP-y will be similarly decorated with fluorescent antibodies, making functional distinction between these overlapping fractions difficult. The analysis may also be skewed if one or more of the VAMPs are present at very different levels of expression.
To address these and other issues, we examined all the VAMPs expressed in 3T3-L1 adipocytes in a systematic manner, avoiding knockdown or genetic deletion, to prevent counterregulatory mechanisms overly complicating our analysis. Our first striking observation is that of all the VAMPs, only VAMPs 2–4 exhibit up-regulation in the fibroblast-to-adipocyte conversion. Although this is not an a priori reason to rule out any protein playing a functional role in the insulin response, several proteins that are involved in insulin-stimulated GLUT4 translocation are up-regulated during differentiation (El-Jack et al., 1999; Shewan et al., 2003; Shi et al., 2008). Quantification of the absolute levels of VAMPs provides further insight. VAMP8 and VAMP3 are the most abundant isoforms in adipocytes, being expressed at approximately twofold and fourfold higher levels than VAMP2, respectively. VAMP4 is the next most abundant; this VAMP has been implicated in GLUT4 sorting but not exocytosis and is expressed at levels approaching those measured for VAMP2. By contrast, VAMP5 and VAMP7 are weakly expressed in these cells. High levels of VAMP3 and VAMP8, known to function in endosomal trafficking, are consistent with the high secretory capacity of fat cells that are known to secrete many adipocytokines. This may also explain why either VAMP can restore insulin-stimulated GLUT4 translocation (in cells devoid of VAMPs 2, 3, and 8), as a significant fraction of GLUT4 is delivered to the cell surface from endosomes; in 3T3-L1 adipocytes, up to 40% of GLUT4 is present within transferrin receptor–positive compartments (Livingstone et al., 1996).
Of interest, we observe different patterns of localization and insulin responsiveness of these different VAMP isoforms. As previously reported, insulin stimulates the delivery of VAMP2 and VAMP3 to the plasma membrane; this is consistent with the notion that insulin triggers delivery of VAMP2-positive GSVs and VAMP3-positive endosomes to the cell surface and that both of these compartments contain GLUT4 (Volchuk et al., 1995; Livingstone et al., 1996; Olson et al., 1997). We did not observe insulin-stimulated delivery of endogenous VAMP8 to the cell surface, a key argument against a functional role for this VAMP in normal adipocyte physiology. When LDM fractions were subfractionated into GSV-enriched and endosome-enriched fractions, all VAMPs were present in both fractions, with the notable and consistent exception of VAMP3, which was predominantly localized within the endosomal fraction. These data, together with the lack of an effect of insulin on VAMP8, strongly suggest that VAMP2 is the main v-SNARE for GSV delivery to the cell surface. This data are supported by coimmunoprecipitation of VAMP2 but no other VAMP with Syntaxin4 (with the exception of VAMP5, which is known to interact with Syntaxin4 in cardiomyocytes; Schwenk et al., 2010).
A combination of the quantification and subcellular fraction data presented here allows us to make some approximations regarding the distribution of VAMP isoforms on GLUT4 vesicles. VAMP2 is expressed at 8.6 × 105 copies/cell and VAMP3 at 1.5 × 106 copies/cell. Sixty percent of these proteins are localized within the LDM fraction (Figure 4) comprising both GSVs and endosomal fractions (Figure 5). VAMP2 is roughly equally split between these two fractions, but VAMP3 is mainly localized within the endosomal fraction (80%; Figure 5). Hence, within the GSV-enriched fraction, there are 241,000 copies of VAMP2 and 133,000 copies of VAMP3; in the endosomal fraction, there are 2.5-fold more copies of VAMP3 than VAMP2. Using a value of 2.8 × 105 copies of GLUT4 per cell (Calderhead et al., 1990) and the data in Figure 5 to distribute this GLUT4 between the GSV and endosomal fractions, we estimate that GSVs contain 4 molecules of VAMP2 and 2 of VAMP per molecule of GLUT4; Kupriyanova et al. (2002) estimated that one GSV contains ∼4 molecules of GLUT4; using this estimate, our data would equate to 16 VAMP2 molecules per GSV, compared with 8 copies of VAMP3. These data compare favorably with the estimates of the number of SNAREs required to catalyze fusion (Weninger et al., 2003; Zhang et al., 2004; Sudhof and Rothman, 2009; Wiederhold and Fasshauer, 2009) but are considerably lower than the estimates of VAMP2 levels on isolated synaptic vesicles (Takamore et al., 2006). This may reflect both biological differences in terms of the kinetics of the secretion event in question (neuronal exocytosis is faster than GLUT4 delivery to the cell surface) and clear oversimplification in our analysis, as it is unlikely that the distributions of these different proteins in the GSV and endosomal fractions are uniform. Nonetheless, the data are consistent with VAMP2 being the major VAMP involved in GSV fusion with the cell surface.
In sum, we provide the first detailed analysis of the cellular content of VAMP isoforms in a single cell type and show that each of these VAMPs can form an SDS-resistant ternary complex with Syntaxin4 and SNAP23. Our data support studies that suggested an important role for VAMP2 in GSV fusion with the plasma membrane and argue against the notion of plasticity in the context of normal cell function. We do not dispute the data presented by Zhao et al. (2009) showing that in the absence of VAMPs 2, 3, and 8, any of these VAMPs alone can reconstitute insulin-stimulated GLUT4 delivery to the cell surface. However, our data are consistent with the notion that in cells not devoid of any VAMP, VAMP2 is the most likely candidate v-SNARE for GLUT4 delivery to the cell surface.
MATERIALS AND METHODS
Cells, antibodies, and plasmid constructs
3T3-L1 fibroblasts were obtained from the American Type Culture Collection (Manassas, VA) and grown and differentiated into adipocytes as outlined (Roccisana et al., 2013). All anti-VAMP antibodies were from Synaptic Systems (Göttingen, Germany), anti-SNAP23 and anti-Syntaxin4 were from Abcam (Cambridge, United Kingdom), and all other antibodies were as described (Roccisana et al., 2013). GST-VAMPs 2 and 3 were as described (Millar et al., 1999; Brandie et al., 2008). GST-VAMPs 4, 5, 7, and 8 were from Andrew Peden (University of Sheffield, Sheffield, United Kingdom).
Recombinant protein expression and purification and SNARE complex assembly assays
Glutathione S-transferase (GST)–tagged VAMP proteins were expressed in BL21(DE3) cells and purified as described (Brandie et al., 2008). Syntaxin4 and SNAP23 were purified exactly as outlined in (Aran et al., 2009), and all proteins were dialyzed against phosphate-buffered saline (PBS) and stored at −80°C until use. For SNARE complex assembly assays, equimolar (∼4 μM) amounts of GST-VAMP protein, histidine (His)-SNAP23, and thrombin-cleaved Syntaxin4 were combined in a volume of 500 μl of PBS supplemented with 100 μg/ml bovine serum albumin and incubated for the indicated time at 4°C with rotation. Reactions were stopped by the addition of 5× Laemmli sample buffer (LSB; 300 mM Tris-Cl, pH 6.8, 10% SDS, 50% glycerol, 25% β-mercaptoethanol, 0.05% bromophenol blue) and immediately heated to 95°C for 5 min. Samples were subjected to SDS–PAGE and immunoblot analysis on the same day as the experiment.
Immunoprecipitation
3T3-L1 adipocytes were treated with 1 mM N-ethylmaleimide and then lysed in immunoprecipitation buffer (50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, pH 7.5, 5 mM EDTA, 10 mM sodium pyrophosphate, 10 mM NaF, 150 mM NaCl, 2 mM β-glycerophosphate, 1 mM dithiothreitol, 1% [vol/vol] Triton X-100, and protease inhibitors) and centrifuged at 12,500 × g for 20 min at 4°C. A 1.5-mg amount of lysate was precleared using protein A beads and then incubated with anti-Syntaxin4 or random rabbit serum for 2 h on ice. Protein A beads were added for a further 2 h and then separated from unbound material by brief centrifugation. Unbound material was retained for analysis and bound material washed three times and eluted using 2× LSB (Sadler et al., 2015).
Subcellular fractionation and immunoblotting
Subcellular fractionation into plasma membrane, high- and low-density membranes, and cytosolic fractions was performed using a well-characterized procedure (Piper et al., 1991; Roccisana et al., 2013); this method routinely underestimates the effect of insulin on GLUT4 translocation to the plasma membrane (Piper et al., 1991; El-Jack et al., 1999; Hashiramoto et al., 2000). Iodixanol gradient centrifugation analysis of freshly isolated LDM fractions was performed as described (Hashiramoto and James, 2000; Maier and Gould, 2000). To quantify the fraction of GLUT4 within the GSV fractions, we expressed the immunoblot signal derived from lanes 1–5 as a fraction of the total GLUT4 signal across all fractions. Total membranes were prepared as described (Roccisana et al., 2013). SDS–PAGE and immunoblotting were performed as described (Roccisana et al., 2013). Immunoblot signals were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Supplementary Material
Acknowledgments
We thank Andrew Peden for cDNAs used in this study. This work was supported by the Diabetes UK Arthur and Sadie Pethybridge Studentship (to J.B.A.S.) and Grant 11/0004289 and support from Diabetes UK to G.W.G. and N.J.B. N.J.B. is a Prize Fellow of the Lister Institute of Preventive Medicine.
Abbreviations used:
- GSV
Glut4-storage vesicles
- PM
plasma membrane
- SNARE
soluble N-ethylmaleimide–sensitive factor attachment protein receptor
- VAMP
vesicle-associated membrane protein.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-09-1368) on December 10, 2014.
REFERENCES
- Aran V, Brandie FM, Boyd AR, Kantidakis T, Rideout EJ, Kelly SM, Gould GW, Bryant NJ. Characterization of two distinct binding modes between syntaxin 4 and Munc18c. Biochem J. 2009;419:655–660. doi: 10.1042/BJ20082293. [DOI] [PubMed] [Google Scholar]
- Bajno L, Peng XR, Schreiber AD, Moore HP, Trimble WS, Grinstein S. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol. 2000;149:697–706. doi: 10.1083/jcb.149.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber JW, Lienhard GE. Isolation of vesicles containing insulin-responsive, intracellular glucose transporters from 3T3-L1 adipocytes. J Biol Chem. 1986;261:16180–16184. [PubMed] [Google Scholar]
- Brandie FM, Aran V, Verma A, McNew JA, Bryant NJ, Gould GW. Negative regulation of syntaxin4/SNAP-23/VAMP2-mediated membrane fusion by Munc18c in vitro. PLoS One. 2008;3:e4074. doi: 10.1371/journal.pone.0004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Gould GW. SNARE proteins underpin insulin-regulated GLUT4 traffic. Traffic. 2011;12:657–664. doi: 10.1111/j.1600-0854.2011.01163.x. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated trafficking of the glucose transporter, Glut4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Calderhead DM, Kitagawa K, Tanner LI, Holman GD, Lienhard GE. Insulin regulation of the two glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1990;265:13800–13808. [PubMed] [Google Scholar]
- Cheatham B, Volchuk A, Kahn CR, Wang L, Rhodes CJ, Klip A. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc Natl Acad Sci USA. 1996;93:15169–15173. doi: 10.1073/pnas.93.26.15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosen-Binker LI, Binker MG, Wang CC, Hong W, Gaisano HY. VAMP8 is the v-SNARE that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis. J Clin Invest. 2008;118:2535–2551. doi: 10.1172/JCI34672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Jack AK, Kandror K, Pilch PF. The formation of an insulin-responsive vesicular cargo compartment is an early event in 3T3-L1 adipocyte differentiation. Mol Biol Cell. 1999;10:1581–1594. doi: 10.1091/mbc.10.5.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduch E, Aledo JC, Watts C, Hundall HS. Proteolytic cleavage of cellubrevin and vesicle-associated membrane protein (VAMP) by tetanus toxin does not impair insulin-stimulated glucose transport or GLUT4 translocation in rat adipocytes. Biochem J. 1997;321:233–238. doi: 10.1042/bj3210233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiramoto M, James DE. Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3-L1 adipocytes. Mol Cell Biol. 2000;20:416–427. doi: 10.1128/mcb.20.1.416-427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson GR, Chamberlain LH, Maier VH, Gould GW. Quantification of SNARE protein levels in 3T3-L1 adipocytes: implications for insulin-stimulated glucose transport. Biochem Biophys Res Commun. 2000;270:841–845. doi: 10.1006/bbrc.2000.2525. [DOI] [PubMed] [Google Scholar]
- Hu C, Hardee D, Minnear F. Membrane fusion by VAMP3 and plasma membrane t-SNAREs. Exp Cell Res. 2007;313:3198–3209. doi: 10.1016/j.yexcr.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi T, Tamori Y, Kanda H, Yoshikawa M, Tateya S, Nishino N, Kasuga M. The t-SNAREs syntaxin4 and SNAP23 but not v-SNARE VAMP2 are indispensable to tether GLUT4 vesicles at the plasma membrane in adipocyte. Biochem Biophys Res Commun. 2010;391:1336–1341. doi: 10.1016/j.bbrc.2009.12.045. [DOI] [PubMed] [Google Scholar]
- Kupriyanova TA, Kandror V, Kandror KV. Isolation and characterization of the two major intracellular Glut4 storage compartments. J Biol Chem. 2002;277:9133–9138. doi: 10.1074/jbc.M106999200. [DOI] [PubMed] [Google Scholar]
- Larance M, Ramm G, Stockli J, van Dam EM, Winata S, Wasinger V, Simpson F, Graham M, Junutula JR, Guilhaus M, et al. Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J Biol Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- Liu Y, Barlowe C. Analysis of Sec22p in endoplasmic reticulum/Golgi transport reveals cellular redundancy in SNARE protein function. Mol Biol Cell. 2002;13:3314–3324. doi: 10.1091/mbc.E02-04-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone C, James DE, Rice JE, Hanpeter D, Gould GW. Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3-L1 adipocytes. Biochem J. 1996;315:487–495. doi: 10.1042/bj3150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier VH, Gould GW. Long-term insulin treatment of 3T3-L1 adipocytes results in mis-targeting of GLUT4: implications for insulin-stimulated glucose transport. Diabetologia. 2000;43:1273–1281. doi: 10.1007/s001250051523. [DOI] [PubMed] [Google Scholar]
- Martin LB, Shewan A, Millar CA, Gould GW, James DE. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3-L1 adipocytes. J Biol Chem. 1998;273:1444–1452. doi: 10.1074/jbc.273.3.1444. [DOI] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar CA, Shewan A, Hickson GR, James DE, Gould GW. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3-L1 adipocytes. Mol Biol Cell. 1999;10:3675–3688. doi: 10.1091/mbc.10.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AL, Knight JB, Pessin JE. Syntaxin 4, VAMP2, and/or VAMP3/Cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1997;17:2425–2435. doi: 10.1128/mcb.17.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera HK, Clarke M, Morris NJ, Hong W, Chamberlain LH, Gould GW. Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes. Mol Biol Cell. 2003;14:2946–2958. doi: 10.1091/mbc.E02-11-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Hess LJ, James DE. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am J Physiol. 1991;260:C570-C580. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- Polgar J, Chung SH, Reed GL. Vesicle-associated membrane protein 3 (VAMP-3) and VAMP-8 are present in human platelets and are required for granule secretion. Blood. 2002;100:1081–1083. doi: 10.1182/blood.v100.3.1081. [DOI] [PubMed] [Google Scholar]
- Randhawa VK, Bilan PJ, Khayat ZA, Daneman N, Liu Z, Ramlal T, Volchuk A, Peng XR, Coppola T, Regazzi R, et al. VAMP2, but not VAMP3/cellubrevin, mediates insulin-dependent incorporation of GLUT4 into the plasma membrane of L6 myoblasts. Mol Biol Cell. 2000;11:2403–2417. doi: 10.1091/mbc.11.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randhawa VK, Thong FS, Lim DY, Li D, Garg RR, Rudge R, Galli T, Rudich A, Klip A. Insulin and hypertonicity recruit GLUT4 to the plasma membrane of muscle cells by using N-ethylmaleimide-sensitive factor-dependent SNARE mechanisms but different v-SNAREs: role of TI-VAMP. Mol Biol Cell. 2004;15:5565–5573. doi: 10.1091/mbc.E04-03-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R, Butler P. Insulin resistance in type II diabetes mellitus. Adv Second Messenger Phosphoprotein Res. 1990;24:511–516. [PubMed] [Google Scholar]
- Roccisana J, Sadler JB, Bryant NJ, Gould GW. Sorting of GLUT4 into its insulin-sensitive store requires the Sec1/Munc18 protein mVps45. Mol Biol Cell. 2013;24:2389–2397. doi: 10.1091/mbc.E13-01-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler JBA, Roccisana J, Virolianen M, Bryant NJ, Gould GW. mVps45 knockdown selectively modulates VAMP expression in 3T3-L1 adipocytes. Commun Integr Biol. 2015 doi: 10.1080/19420889.2015.1026494. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Schwenk RW, Dirkx E, Coumans WA, Bonen A, Klip A, Glatz JF, Luiken JJ. Requirement for distinct vesicle-associated membrane proteins in insulin- and AMP-activated protein kinase (AMPK)-induced translocation of GLUT4 and CD36 in cultured cardiomyocytes. Diabetologia. 2010;53:2209–2219. doi: 10.1007/s00125-010-1832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd PR, Kahn BB. Glucose transporters and insulin action. N Engl J Med. 1999;341:248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- Shewan AM, van Dam EM, Martin S, Luen TB, Hong W, Bryant NJ, James DE. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in syntaxins 6 and 16 but not TGN38: Involvement of an acidic targeting motif. Mol Biol Cell. 2003;14:973–986. doi: 10.1091/mbc.E02-06-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Huang G, Kandror KV. Self-assembly of Glut4 storage vesicles during differentiation of 3T3-L1 adipocytes. J Biol Chem. 2008;283:30311–30321. doi: 10.1074/jbc.M805182200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamore S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Volchuk A, Sargeant R, Sumitani S, Liu Z, He L, Klip A. Cellubrevin is a resident protein of insulin-sensitive GLUT4 glucose transporter vesicles in 3T3-L1 adipocytes. J Biol Chem. 1995;270:8233–8240. doi: 10.1074/jbc.270.14.8233. [DOI] [PubMed] [Google Scholar]
- Weninger K, Bowen ME, Chu S, Brunger AT. Single-molecule studies of SNARE complex assembly reveal parallel and antiparallel configurations. Proc Natl Acad Sci USA. 2003;100:14800–14805. doi: 10.1073/pnas.2036428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold K, Fasshauer D. Is assembly of the SNARE complex enough to fuel membrane fusion. J Biol Chem. 2009;284:13143–13152. doi: 10.1074/jbc.M900703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D, Pessin JE. Mapping of R-SNARE function at distinct intracellular GLUT4 trafficking steps in adipocytes. J Cell Biol. 2008;180:375–387. doi: 10.1083/jcb.200709108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Gonzalez L, Jr, Prekeris R, Steegmaier M, Advani RJ, Scheller RH. SNARE interactions are not selective. Implications for membrane fusion specificity. J Biol Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]
- Zhang F, Chen Y, Su Z, Shin Y-K. SNARE assembly and membrane fusion, a kinetic analysis. J Biol Chem. 2004;279:38668–38672. doi: 10.1074/jbc.M404904200. [DOI] [PubMed] [Google Scholar]
- Zhao P, Yang L, Lopez JA, Fan J, Burchfield JG, Bai L, Hong W, Xu T, James DE. Variations in the requirement for v-SNAREs in GLUT4 trafficking in adipocytes. J Cell Sci. 2009;122:3472–3480. doi: 10.1242/jcs.047449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.