Abstract
Rationale
Given the increasing abuse of prescription opioids, particularly in adolescents, surprisingly few preclinical studies have explored effects of opioids in adolescents (versus adults).
Objectives
This study compared the conditioned rewarding effects of morphine, without (experiment 1) and with morphine pre-exposure (experiment 2), in adolescent and adult male mice.
Methods
Experiment 1: on each of three consecutive days, one of the two conditioning sessions was preceded by an injection of a particular dose of morphine (0.1, 0.32, 1, 3.2, 10, 32, or 100 mg/kg, i.p.) and the other by saline; place preference was tested on day 4. Experiment 2: mice received once daily injections of saline or a particular dose of morphine (17.8 or 56 mg/kg) for 4 days, and 3 days later, place conditioning with morphine (0.32, 1, 3.2, or 10 mg/kg) began.
Results
In both experiments, morphine induced conditioned place preference along similar inverted U-shaped dose-response curves in adolescent and adult mice, with maximal effects between 0.32–10 mg/kg. Morphine pre-exposure did not sensitize morphine-induced conditioned place preference; instead, tolerance occurred, but only in adults. Adolescents were more sensitive than adults to morphine-induced locomotor stimulation. Response to novelty predicted the locomotor stimulating effects of morphine in adolescents, but not its rewarding effects.
Conclusions
The rewarding effects of morphine were similar in adolescent and adult mice, but showed differential tolerance after morphine pre-exposure. Adolescents were more sensitive than adults to the acute locomotor stimulating effects of morphine, consistent with dopamine systems involved in locomotor activity being overactive during adolescence.
Keywords: Morphine, Conditioned Place Preference, Locomotion, Sensitization, Tolerance, Withdrawal, Adolescent, Adult, Mouse
The increased prescription of opioids to manage chronic pain has been accompanied by a dramatic rise in opioid abuse that now is a major public health concern (Compton and Volkow 2006). Opioid analgesic abuse is particularly problematic for adolescents, because of uncertain implications of exposure to opioids during adolescence for future addiction. The abuse of opioid analgesics, like that of other drugs, is mostly concentrated in adolescents and young adults (Substance Abuse and Mental Health Services Administration 2003), yet little is known about opioid effects in these age groups. Most of what we know about opioid abuse and addiction has been learned from heroin addiction in 20 to 40-year-old individuals, and from research in adult animals.
Like most drugs of abuse, opioids stimulate the mesolimbic dopamine system in the brain. Mesolimbic and nigrostriatal dopamine systems appear to be overactive during adolescence (Wahlstrom et al. 2010), which could underlie the different balance of rewarding and aversive effects of drugs of abuse in adolescents compared with adults (Schramm-Sapyta et al. 2009): adolescents may be more sensitive to the rewarding effects of drugs and less sensitive to withdrawal effects. Evidence for this differential sensitivity has been obtained in animal models with nicotine, ethanol, THC, amphetamine, and cocaine. However, only a few studies have compared dopamine-related effects of opioids in adolescent and adult rats (for example, see Campbell et al. 2000; White and Holtzman 2005; White et al. 2008; Doherty et al. 2009; Doherty and Frantz 2012, 2013) or in adolescent or adult mice (for example, see Hodgson et al. 2009; Zhang et al. 2009; Niikura et al. 2013).
The present study is part of an effort to examine dopamine-related behavioral effects of morphine in adolescent and adult mice. Previously, acute administration of morphine was found to stimulate locomotion to higher maximal levels in adolescents than in adults (Koek et al., 2012). Morphine stimulates the mesolimbic dopamine system indirectly by inhibiting GABAergic interneurons in the ventral tegmental area that inhibit dopaminergic neurons (Johnson and North, 1992). Overactivity of the mesolimbic dopamine system during adolescence (Wahlstrom et al., 2010) conceivably underlies age differences in the acute effects of morphine on locomotion (Koek et al., 2012). When administered repeatedly, the locomotor-stimulating effects of many drugs of abuse are enhanced; this sensitization is thought to reflect long-lasting brain changes that are hypothesized to cause addiction (e.g., Robinson and Berridge 2003). However, imaging studies have shown a blunted dopamine response, or the opposite of sensitization, in cocaine dependent humans (Narendran and Martinez 2008; see Robinson and Berridge 2008 for an alternative explanation of the imaging results). In a recent review of sensitization in rodents, primates, and humans, Vanderschuren and Pierce (2010) conclude that sensitization is involved in certain, but not all, phases of drug addiction, and is an important initial step in the addiction process. Repeated administration of morphine is known to produce locomotor sensitization in adult rodents (e.g., Kalivas and Duffy 1987; Kuribara 1996; Vanderschuren et al. 1997). Consistent with previous findings in rats (White and Holtzman 2005; White et al. 2008), morphine-induced locomotor sensitization during adulthood is more pronounced in mice repeatedly exposed to morphine as adolescents than as adults (Koek, 2013), conceivably involving overactivity of the mesolimbic dopamine system during adolescence.
Mu-opioid receptors in the ventral tegmental area near the cell bodies of mesolimbic dopamine neurons are not only involved in morphine-induced locomotion (Joyce and Iversen 1979) but also in other effects of morphine, such as conditioned place preference (Phillips and LePiane 1980). Activation of these receptors stimulates dopamine transmission preferentially in the mesolimbic system (Di Chiara and Imperato 1988). If morphine stimulates locomotor activity in adolescents through a normal opioid system [mu-opioid receptor function assessed by GTPgammaS binding does not differ between adolescents and adults (Talbot et al. 2005)] coupled to an overactive mesolimbic dopamine system, adolescents would be expected to be more sensitive also to other effects of morphine mediated by the mesolimbic dopamine system, such as its rewarding effects. Conditioned place preference is often used to assess motivational effects of drugs (for reviews, see Tzschentke 1998, 2007), including opioids (e.g., Shippenberg and Herz 1987; Vezina and Stewart 1987). To date, only two studies directly compared opioid-induced conditioned place preference in adolescent and adult rodents. Campbell et al. (2000) found no evidence of reliable differences between adolescent and adult rats in place preference for the morphine doses that were tested (i.e., 1 and 2.5 mg/kg). Adult mice developed conditioned preference to the lowest dose of oxycodone tested (0.3 mg/kg), but adolescent mice did not (Niikura et al., 2013). Studies directly comparing morphine-induced conditioned place preference in adolescent and adult mice are lacking.
Preliminary data obtained in our laboratory in adult mice suggested that morphine produced place preference at low and intermediate doses and place aversion at high doses. To characterize this inverted-U shaped dose-response curve, and to examine if it had a similar shape in adolescents, the first experiment reported here studied morphine-induced conditioned place preference over a broad range of doses in adolescents and adults. The second experiment examined the effects of repeated exposure to morphine on morphine-induced conditioned place preference in adolescents and adults. In adult rats, such pre-exposure has been reported to produce sensitization to the rewarding effects of morphine (e.g., Shippenberg et al. 1996, 1998). Locomotor activity in a novel environment, assessed in the present study before conditioning started, was used to examine if differences in response to novelty predict morphine-induced locomotor stimulation, sensitization, and conditioned place preference, based on the hypothesis that an individual’s response to novelty predicts its response to drugs of abuse (Piazza et al. 1990).
Methods
Subjects
The present study examined male C57BL/6J mice between postnatal days 28–42, described as prototypical adolescence by Spear (2000), in comparison with adult mice (postnatal day 64–78). Mice were obtained by breeding C57BL/6J mice from the Jackson Laboratory (Bar Harbor, ME). Housing and rearing conditions were identical to those described in Koek et al. (2012). Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the Guide for the Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/Guide-for-thecare-and-use-of-laboratoryanimals. pdf).
Procedures
The equipment used to study conditioned place preference was similar to that described previously (Cunningham et al. 1992) and consisted of eight 30×15×15 cm customized acrylic boxes (Instrumentation Services, University of Texas Health Science Center, San Antonio) that were separately enclosed in commercially available sound-attenuating chambers (model no. ENV-022M; MED Associates, St. Albans, VT). Between sessions, the floor and inside of the boxes were wiped, and the litter paper beneath the floor was changed. The metal floors of the boxes were removable and varied in texture across conditions. For half of the mice, morphine was paired with the hole floor-texture (evenly distributed 6.4 mm round holes on 9.5 mm staggered centers) and the vehicle with the grid floor-texture (parallel 2.3-mm stainless steel rods mounted 6.4 mm apart); pairings were opposite for the remaining mice. On the preference test day, half (15 × 15 cm) of the floor had the hole texture and half had the grid texture. Location in the chamber and horizontal activity were measured with four infrared light beams spaced 6 cm apart and located 2 cm above the floor of each box. Occlusions of the infrared light beams were counted using commercially available computer software (Multi-Varimex version 2.10, Columbus Instruments, Columbus, OH).
The conditioned place preference procedure was similar to that described by Cunningham et al. (1992, 1999) and consisted of three phases: habituation (one session), conditioning (six sessions), and place preference test (one session). Mice received an i.p. injection immediately before each session, and were placed in the center of the apparatus. The habituation session was intended to reduce the novelty and stress associated with handling, injection, and exposure to the apparatus: all mice received saline and were placed in the apparatus for 30 min on a floor covered with paper. The following three days, conditioning sessions were held. Conditioning consisted of pairing one floor type with the injection of morphine and the other floor type with the injection of saline. To be able to examine effects of repeated pre-exposure to morphine on morphine-induced conditioned place preference (experiment 2, see below) within the two-week period (postnatal days 28–42) commonly described as prototypical adolescence (Spear, 2000), thirty-min conditioning sessions were conducted twice per day, once after morphine and once after saline, with 6–8 h between the sessions, and with the order of morphine and saline sessions counterbalanced between and within animals. The floor preference test was given on the day after the last conditioning day. All mice received a saline injection immediately before the 30-min preference test. The percentage of time spent on the morphine-paired floor was used to measure place preference.
Previous reports (e.g., Cunningham et al., 1992) and preliminary data obtained in our laboratory showed that adult and adolescent C57BL/6J control mice repeatedly treated only with saline spent the same amount of time on both floor types during the preference test, indicating equal preference for the “hole” and the “grid” floors, and allowing the use of an unbiased method to assess conditioned place preference. In an effort to confirm these observations and to extend them to the conditions of the present study, experiment 1 not only involved different groups of adult and of adolescent mice (n=8–12 per group) that received a particular dose of morphine (i.e., 0.1, 0.32, 1, 3.2, 10, 32, or 100 mg/kg) or saline, but also a control group of adult mice and a control group of adolescent mice that received only saline (n=16 per group).
Sensitization to morphine-induced place preference was examined in experiment 2 with a procedure similar to that described by Shippenberg et al. (1996). Different groups of mice received four consecutive daily injections with saline, 17.8, or 56 mg/kg morphine. Immediately after each injection, mice were returned to their home cage. Three days after the fourth injection, morphine-induced conditioned place preference was examined with the same procedure that was used in experiment 1 (n=8–12 per group). Previously, 17.8 mg/kg was found to be the lowest dose of morphine that produced locomotor sensitization in adult and adolescent C57BL/6J mice (Koek et al., 2013). Experiment 2 examined conditioned place preference at doses of morphine (i.e., 0.32–10 mg/kg) found to be effective in experiment 1.
Data analyses
All analyses were conducted with GraphPad Prism version 6.03 for Windows (GraphPad Software, La Jolla, CA, USA), except repeated measures ANOVA, which were conducted using NCSS 9 for Windows (NCSS, Kaysville, UT, USA) to correct for possible violations of sphericity by means of the Geisser-Greenhouse adjustment. Statistical significance was defined as p<0.05. In experiment 1, morphine-induced conditioned place preference, measured as the percentage time spent on the morphine-paired floor, was analyzed by two-factor ANOVA with dose (0–100 mg/kg) and age (adolescent, adult) as between-subjects factors. In experiment 2, age-related differences in animals pre-exposed to repeated saline were analyzed by two-factor ANOVA with conditioning dose (0–100 mg/kg) and age (adolescent, adult) as between-subjects factors, and the effects of morphine pre-exposure on morphine-induced conditioned place preference were analyzed separately for each age group by two-factor ANOVA with pre-exposure dose and conditioning dose as between-subjects factors.
There were significant baseline differences in locomotion among the age groups upon initial exposure to the activity chambers (see Results), consistent with previous observations (Koek et al., 2012; Koek, 2013). Therefore, locomotion during morphine conditioning sessions was expressed for each animal as a percentage of locomotion during the saline conditioning session conducted the same day [for additional details of this approach, see results and discussion in Koek et al. (2012)]. Locomotion data obtained during the first morphine conditioning session were analyzed by two-factor ANOVA (conditioning dose, age) in experiment 1. In experiment 2, age-related differences in animals pre-exposed to repeated saline were analyzed by two-factor ANOVA with conditioning dose and age as between-subjects factors, and the effects of morphine pre-exposure were analyzed separately for each age group by two-factor ANOVA with pre-exposure dose and conditioning dose as between-subjects factors. Changes in locomotion during the conditioning phase were analyzed separately for each conditioning dose by two-factor ANOVA with age as between-subjects factor and morphine session as within-subjects factor in experiment 1, and by two-factor ANOVA with pre-exposure dose as between-subjects factor and morphine session as within-subjects factor in experiment 2. Multiple comparisons were conducted with Holm-Sidak's test, implemented in GraphPad Prism.
Relations between locomotor activity during the habituation session and morphine-induced locomotion and conditioned place preference were examined by the Pearson product-moment correlation coefficient and linear regression. Differences between independent correlation coefficients were tested as described in Cohen and Cohen (1983), and differences between slopes and intercepts of regression lines were analyzed by the F ratio test in GraphPad Prism.
Drugs
Morphine sulfate (National Institute on Drug Abuse, Research Technology Branch, Research Triangle Park, NC) was dissolved in physiological saline and injected i.p. in a volume of 10 ml/kg.
Results
Experiment 1
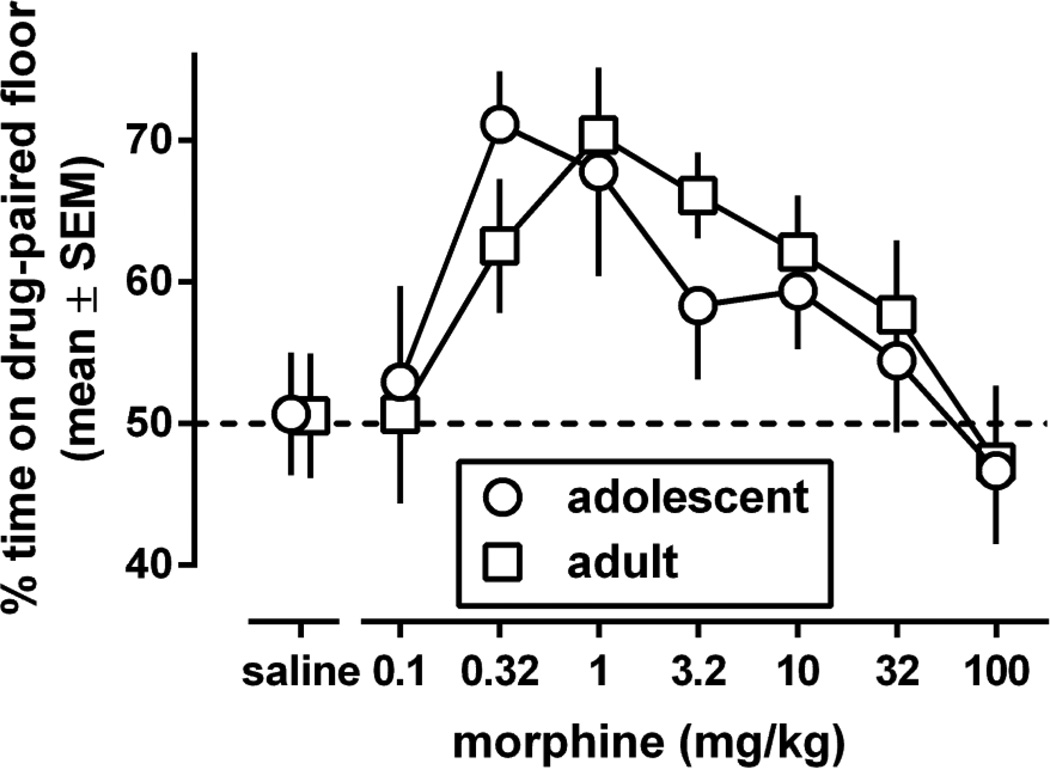
Adolescent and adult C57BL/6J control mice repeatedly treated only with saline spent the same amount of time on both floor types during the preference test [% time spent on the grid floor (mean, 95% CL): 50.7 (41.4–60) and 50.5 (41.1–60) in adolescents and adults, respectively; Fig. 1, saline data points]. Morphine dose-dependently induced conditioned place preference, and did so similarly in adolescent and in adult mice [dose: F(7,156)=4.70, p<0.0001; age: p=0.78; dose × age: p=0.90]. In both age groups the dose-response curve of morphine showed an initial rise, attained an apparent maximum, and returned to saline levels as the dose was increased further. Averaged across age groups, morphine significantly increased the percentage time on the drug-paired floor at 0.32, 1, 3.2, and 10 mg/kg compared with saline (p=0.0017, 0.0004, 0.011, 0.028, respectively) to an apparent maximum of 69 (95% CL:60–78) at 1 mg/kg.
Fig. 1.
Morphine-induced conditioned place preference (% time on the morphine-paired floor for all animals except those trained only with saline, for which % time on the grid floor was determined) in adolescent and adult male C57BL/6J mice (experiment 1). Results are shown as mean (± SEM) values (n=8–12).
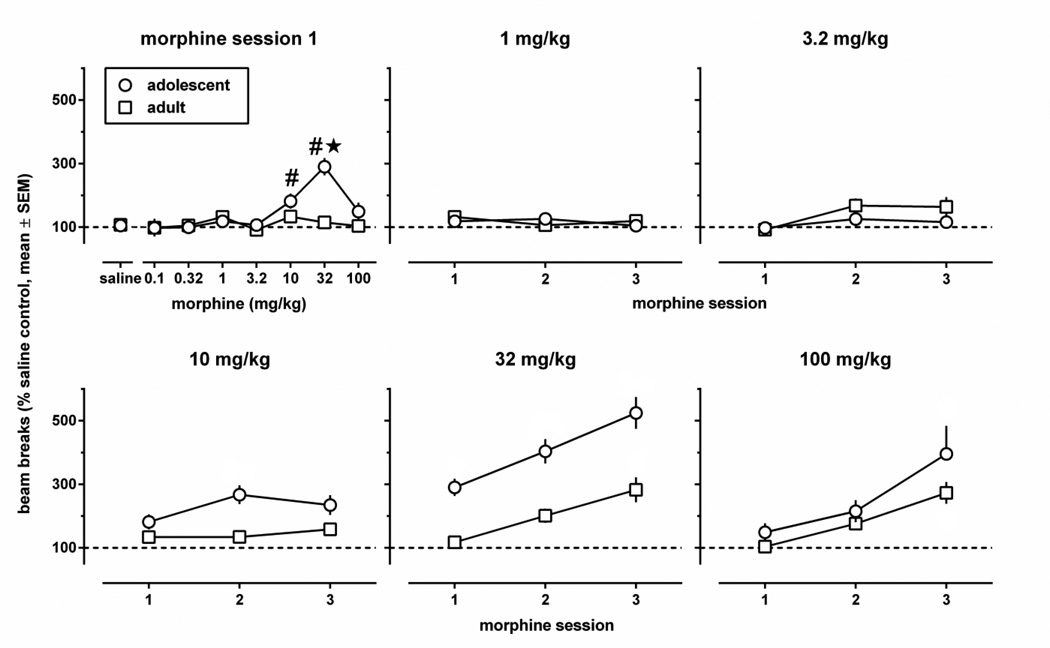
During the initial 30 min exposure to the activity chambers on day 1, basal locomotion was significantly lower in adolescents compared with adults: mean beam breaks (95% confidence limits) were 931 (861–1000) and 1376 (1316–1437), respectively. Because of these age-related differences, drug effects on locomotion during morphine training sessions were expressed for each animal as a percentage of locomotion during the saline training session that was conducted the same day. During the first morphine training session, morphine stimulated locomotion in a dose- and age-dependent manner (Fig. 2, upper left panel) [dose: F(7,138)=8.66, p<0.0001; age: F(1,138)=12.15, p<0.0001; dose × age F(7,138)=6.47, p<0.0001]. Morphine stimulated locomotion only in adolescents, and only at 10 and 32 mg/kg. When these doses were administered repeatedly, they continued to stimulate locomotion more in adolescents than in adults, and they stimulated locomotion more than during the first morphine training session in a manner that did not interact with age (Fig. 2, lower panels) [ANOVA 10 mg/kg data; age: F(1,19)=15.09, p=0.001; session, p=0.12; age × session: p=0.17; ANOVA 32 mg/kg data; age: F(1,21)=46.50, p<0.0001; session: F(2,42)=18.18, p<0.0001; age × session: p=0.56]. Locomotor sensitization was apparent also at 100 mg/kg, and was similar in both age groups [session: F(2,42)=18.97, p<0.0001; age, p=0.14; age × session: p=0.39]. A single administration of 32 or 100 mg/kg morphine was sufficient to induce locomotor sensitization: for each of these doses, beam breaks averaged across age groups were significantly higher during the second and the third session than during the first session (32 mg/kg: session 2 vs session 1, p=0.0046, session 3 vs session 1, p<0.0001; 100 mg/kg: session 2 vs session 1, p=0.049, session 3 vs session 1, p<0.0001). Repeated administration of doses lower than 10 mg/kg did not significantly increase locomotion in either age group (Fig. 3, upper middle and upper right panels for 1 and 3.2 mg/kg, respectively; data not shown for doses lower than 3.2 mg/kg).
Fig. 2.
Locomotor activity after an i.p. injection of morphine or its vehicle in adolescent and adult male C57BL/6J mice during the first morphine place conditioning session (upper left panel) and during all three morphine conditioning sessions (all other panels) for doses 1–100 mg/kg (experiment 1). Results are shown as mean (± SEM) values. Error bars that are not shown are contained within the symbol. Locomotion during morphine conditioning sessions was expressed for each animal as a percentage of locomotion during the saline training session that was conducted the same day. Asterisks indicate p<0.05 compared with adults, and pound signs indicate p<0.05 compared with saline.
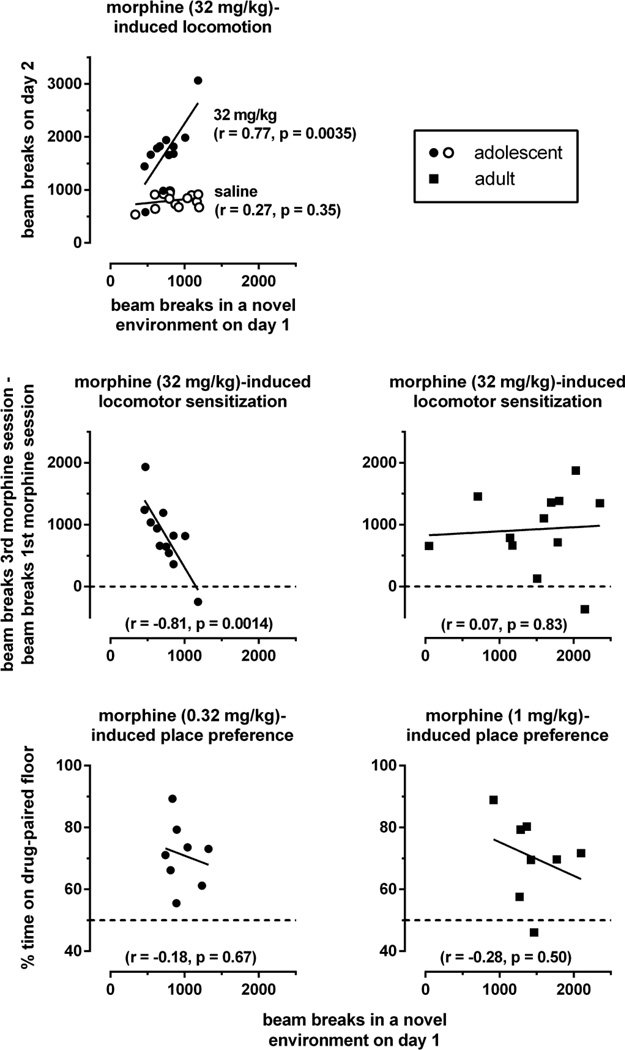
Fig. 3.
Relation between response to novelty on day 1 and morphine-induced locomotion on day 2 (upper left panel), morphine-induce sensitization of locomotion on days 2–4 (middle panels), and morphine-induced conditioned place preference on day 5 (lower panels) (experiment 1). Data points represent values obtained in individual mice, lines are best fitting regressions, and “r” is Pearson’s correlation coefficient.
Beam breaks during the first exposure to the conditioning apparatus on day 1 were used as a measure of locomotor response to novelty, and locomotion during the first morphine-pairing session was used as a measure of the acute locomotor-activating effects of morphine. Morphine acutely stimulated locomotion only in adolescents, with maximal effects at 32 mg/kg (Fig. 2, upper left panel). At this dose, locomotion varied markedly among animals: the total number of beam breaks ranged from about 500 to more than 3000 (Fig. 3, upper left panel, filled circles). These differences in the acute locomotor-stimulating effects of morphine correlated significantly and positively with differences in the locomotor response to novelty. A similar correlation was not apparent in animals treated with saline on day 2 (upper left panel, open circles). The difference between the correlation coefficients obtained in the adolescents treated with morphine and in the adolescents treated with saline approached statistical significance (p=0.099), and the difference between the slopes of the regression lines [i.e., 2.1 (0.56) (mean and SEM) and 0.14 (0.15) in animals treated on day 2 with 32 mg/kg morphine or saline, respectively] was statistically significant [F(1,22)=15.24, p<0.001]. At a dose of 10 mg/kg, which stimulated locomotion in adolescents, but less extensively than 32 mg/kg (Fig. 2, upper left panel), the correlation coefficient between the acute locomotor-stimulating effects of morphine and the locomotor response to novelty was positive (r=0.38) but not statistically significant (p=0.31, data not shown).
Repeated administration of morphine during the conditioning phase of experiment 1 increased its locomotor-stimulating effects in adolescents and in adults, particularly at 32 mg/kg (Fig. 2). Locomotor sensitization observed with this dose of morphine, measured by the increase of the number of beam breaks from the 1st to the 3rd morphine conditioning session, varied between a minimum close to 0 and a maximum of almost 2000 in both age groups (Fig. 3, middle panels). Differences in sensitization correlated significantly with differences in the locomotor response to novelty in adolescents (r=−0.81), but not in adults (r=0.07). These correlation coefficients differed significantly from each other (p=0.011), as did the slopes of the regression lines [i.e., −2.01 (0.46) and 0.14 (0.15) in adolescents and in adults, respectively; F(1,20)=7.32, p=0.014]. Thus, in adolescents, but not in adults, low levels of locomotion in a novel environment appear to be associated with high levels of morphine-induced locomotor sensitization. Note, however, that the absolute number of beam breaks observed in adolescents during the 3rd morphine conditioning session did not correlate (r=0.12, p=0.71) with the response to novelty.
Although the locomotor response to novelty appeared to predict the effects of acute and repeated administration of morphine on locomotion in adolescents, it did not predict morphine-induced conditioned place preference in adolescents or adults (Fig. 3, lower panels). In both age groups, place preference at effective doses in experiment 1 (i.e., 0.32 mg/kg in adolescents, and 1 mg/kg in adults) varied between a minimum of about 50% and a maximum of almost 90%. This variation was unrelated to the locomotor response to novelty: the correlation coefficients of −0.18 in adolescents and −0.28 in adults were not statistically significant and did not differ significantly from each other.
Experiment 2
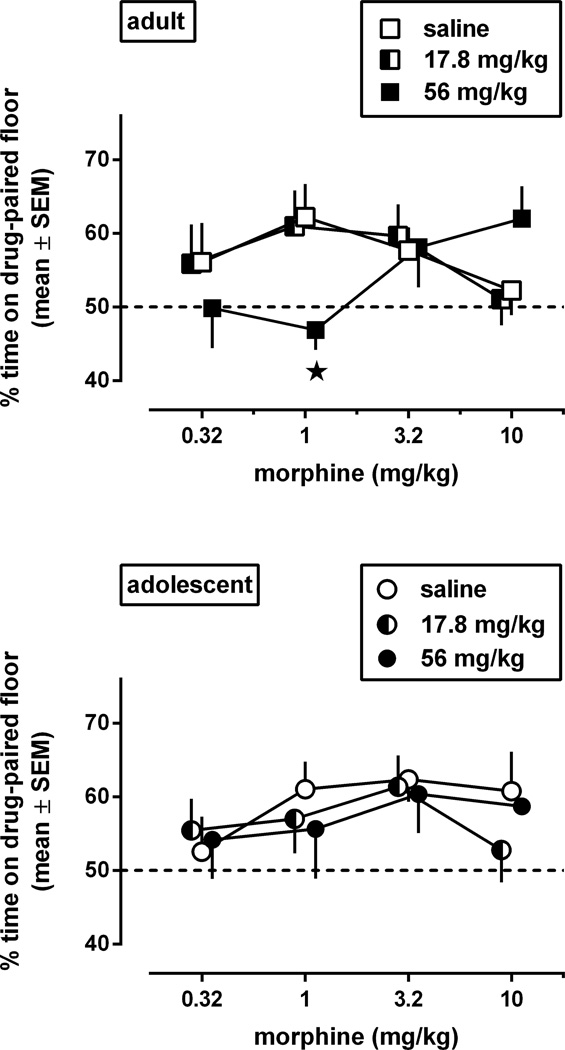
Morphine induced conditioned place preference similarly in adult and adolescent mice repeatedly treated with saline in the home cage before place conditioning started (Fig. 4, open symbols) (age: p=0.48; age × conditioning dose: p=0.49). Averaged across age groups, the mean preference values in saline pre-exposed animals conditioned with 1 and 3.2 mg/kg morphine differed significantly from 50% (p=0.0004 and 0.0036, respectively), whereas the values obtained with 0.32 and 10 mg/kg did not (p=0.31 and 0.11, respectively) (one sample t tests with Holm-Sidak's correction for multiple comparisons). In saline pre-exposed animals, morphine increased the percentage time on the drug-paired floor to a maximum of 60 (95% CL:54–66) at 1 mg/kg, which did not differ significantly from the maximum percentage obtained in experiment 1 in animals not pre-exposed to saline [i.e., 69 (60–75)].
Fig. 4.
Morphine-induced conditioned place preference in adult (upper panel) and adolescent (lower panel) male C57BL/6J mice after pre-exposure to morphine (four daily injections, ending three days before conditioning started) (experiment 2). Results are shown as mean (± SEM) values (n=8–12). Error bars that are not shown are contained within the symbol. Asterisks indicate p<0.05 compared with results in mice pre-exposed to saline.
Pre-exposure to morphine did not enhance morphine-induced conditioned place preference. Instead, pre-exposure to 56 mg/kg morphine, but not to 17.8 mg/kg morphine, shifted the morphine dose-response curve to the right in adults [the pre-exposure dose × conditioning dose interaction approached statistical significance: F(6,155)=1.82, p=0.10; pre-exposure dose: p=0.61]. In adolescents, morphine pre-exposure did not significantly affect morphine-induced conditioned place preference [pre-exposure dose × conditioning dose: p=0.96: pre-exposure dose p=0.61].
Morphine increased locomotion only at the highest conditioning dose tested (i.e., 10 mg/kg), and did so in a manner that generally did not depend on age, conditioning session, or morphine pre-exposure, except that morphine pre-exposure significantly decreased 10 mg/kg morphine-induced locomotion in adults [F(2,33)=3.67, p=0.036], but not in adolescents (p=0.66) (data not shown). As in experiment 1, the locomotor-stimulating effects of 10 mg/kg morphine in adolescents did not correlate significantly with the locomotor response to novelty (r=0.11, p=0.79). Also consistent with experiment 1, conditioned place preference induced by 1 mg/kg morphine in experiment 2 was unrelated to the locomotor response to novelty: the correlation coefficients of −0.08 in adolescents and −0.44 in adults were not statistically significant and did not differ significantly from each other.
Discussion
Confirming and extending previous findings in mice (Koek et al. 2012; Koek 2013) and rats (Spear et al. 1982; White et al. 2008), adolescents were more sensitive to the acute locomotor-stimulating effects of morphine than adults. Morphine stimulates the mesolimbic dopamine system indirectly by inhibiting GABAergic interneurons in the ventral tegmental area that inhibit dopaminergic neurons (Johnson and North 1992). The adolescent brain undergoes extensive changes, including changes in the mesolimbic dopamine system (Wahlstrom et al. 2010). The activity of ventral tegmental dopamine neurons peaks during adolescence, potentially because GABA tone increases as adulthood is reached (McCutcheon et al. 2012). This increased activity of ventral tegmental dopamine neurons during adolescence relative to adulthood conceivably underlies the age-related effects of morphine on locomotion.
If morphine stimulates locomotor activity in adolescents by indirectly stimulating an overactive mesolimbic dopamine system, adolescents would be expected to be more sensitive also to other effects of morphine that are mediated indirectly through this dopamine system, such as its rewarding effects. However, in both experiments reported here, morphine-induced conditioned place preference in adolescents did not differ significantly from that in adults. These results are consistent with the finding that morphine-induced conditioned place preference in adolescent rats was similar in magnitude to that shown by adults (Campbell et al. 2000), but contrast with a report that the prescription opioid oxycodone was about 3-fold less potent to produce conditioned place preference in adolescent than in adult mice (Niikura et al. 2013), and with a report that adolescent rats did not exhibit morphine-induced conditioned place preference (Bolanos et al. 1996). Compared with adults, adolescents self-administered less morphine (Doherty et al. 2009) and less oxycodone (Zhang et al. 2009), which could reflect a higher sensitivity to the reinforcing effects of opioids in adolescents. However, a more recent study (Doherty and Frantz 2012) failed to find evidence for robust age differences in the acute reinforcing effects of heroin. Clearly, further studies are needed to delineate the conditions under which the rewarding effects of opioids depend on age.
In contrast with the rewarding effects of morphine, which in the present study were similar in adolescent and adult mice, the acute locomotor stimulating effects of morphine were greater in adolescent than in adult mice. These findings argue against the notion that the mesolimbic dopamine system similarly mediates locomotor activity and reward. Instead, they suggest the possibility that an overactive mesolimbic dopamine system in adolescents enhanced morphine-induced locomotion but not conditioned place preference, because the latter effect may be less dependent on the mesolimbic dopamine system than the former. This possibility is consistent with data in dopamine-deficient mice showing that dopamine is a crucial component of morphine-induce locomotion, but may not always be required for morphine-induced reward as measured by conditioned place preference (Hnasko et al. 2005).
In the present study, the conditioned place preference-inducing effects of morphine were examined over a range of doses larger than often used in conditioned place preference studies in rodents (Tzschentke 1998, 2007), and an inverted dose-response function was obtained in adolescent and adults. The interval between a drug and a subsequent saline session was 6–8 h when a drug session was conducted in the morning and was 16–18 h when a drug session was conducted in the afternoon. Because the biological half-life of morphine in mouse brain is estimated to be 1 h (Ishikawa et al. 1983), the concentration remaining at 6–8 and 16–18 h would be about 1 and 0.01 percent of the original concentration, respectively. While these washout periods may be sufficient for the elimination of low to moderate doses of morphine (i.e., 0.1–10 mg/kg), concentrations of morphine 6–8 h after high doses (32 and 100 mg/kg) may be similar to those acutely produced by 100-fold lower, but still behaviorally active, doses (i.e., 0.32 and 1 mg/kg). Conceivably, the presence of drug during the vehicle conditioning session, albeit at a much lower concentration, could disrupt the conditioning. Thus, one may expect less conditioning under these conditions, especially if such disruption would occur more often. However, the place preference results obtained with 32 and 100 mg/kg morphine were not significantly affected by whether the sequence of conditioning sessions included one or two saline sessions within 6–8 h after a drug session (data not shown). This suggests that if interference occurred, a single affected session is sufficient to produce it. Alternatively, high doses of morphine may be less rewarding than low doses. To discriminate between these possibilities, the washout period between drug administration and the vehicle conditioning sessions should be prolonged, as was done, for example, to examine conditioned place preference produced by high doses of the long-acting opioid buprenorphine (Tzschentke 2004).
The locomotor-stimulating effects of 32 and 100 mg/kg morphine increased during repeated administration in the first experiment, consistent with other findings of locomotor sensitization after administration of morphine in rats (Vanderschuren et al. 2001) and mice (Valjent et al. 2010; Luo et al. 2011; Koek 2013). These short-term effects of repeated administration of morphine on locomotion were similar in adolescents and adults, confirming and extending previous observations (Koek 2013). In the second experiment, locomotor sensitization occurred neither in adolescents nor in adults after repeated administration of the highest training dose (i.e., 10 mg/kg), in agreement with a lack of locomotor sensitization observed with 10 mg/kg morphine in experiment 1. Surprisingly, morphine pre-exposure did not sensitize morphine-induced conditioned place preference; instead, tolerance occurred, but only in adults. This finding contrasts with reports in adult rats that morphine pre-exposure produced sensitization to the rewarding effects of morphine (e.g., Shippenberg et al. 1996, 1998). These latter studies used a pre-exposure dose of morphine (i.e., 5 mg/kg) lower than those used in the present studies (17.8 and 56 mg/kg), which were selected because of their ability to produce intermediate to maximal locomotor sensitization (Koek 2013). Thus, a more detailed characterization of the effects of morphine pre-expose on morphine place conditioning in mice awaits studies with lower doses than were used in the present experiments. Meanwhile, the results suggest that tolerance to the rewarding effects of morphine occurred in adults, but not in adolescents. However, antinociceptive tolerance to morphine tended to be greater in adolescent than in adult rats (Ingram et al. 2007). It is presently unknown if such differential tolerance in adults and adolescents extends to other effects of morphine.
An individual’s response to novelty has been postulated to predict its response to drugs of abuse and particularly to their addictive properties (Piazza et al. 1990). This hypothesis is supported by studies reporting correlations between responses to a novel environment and various drug effects; however, such studies are sometimes hampered by methodological and statistical weaknesses such as the lack of consideration for correlation in the control group and the calculation of spurious correlations (Quertemont et al. 2004). In experiment 1, the acute locomotor stimulating effects of morphine in adolescents correlated positively with locomotor activity during the first exposure to the test apparatus, used to measure response to novelty. A similar positive correlation was not apparent in controls treated with saline, indicating that the positive correlation was not due to animals showing a stable pattern of activity whatever injection they received. Morphine-induced locomotor sensitization, measured as the difference between locomotion during the first and the last morphine session, which is not intrinsically related to locomotion during the first exposure to the test apparatus, was negatively correlated with response to novelty in adolescents, but was unrelated in adults. Conceivably, this negative correlation could be due to morphine-stimulated locomotion reaching a ceiling in adolescents. This seems unlikely because under similar conditions (Koek 2013) adolescents have shown number of beam break values almost twice as large as the maximal values observed in the present study. However, because morphine-induced locomotor sensitization reaches a maximum (Koek 2013), the negative correlation between response to novelty and the increase of locomotor activity from the 1st to the 3rd morphine session could also result from an initially low drug response allowing a larger increase to maximum than an initially high drug response. The positive correlation between response to novelty and locomotion during the 1st morphine session, and the lack of correlation between response to novelty and locomotion during the 3rd morphine session are consistent with this interpretation. Morphine-induced conditioned place preference could not be predicted by response to novelty, neither in adolescents nor in adults. This contrasts with the finding in rats that high novelty seekers showed increased place preference with 5 mg/kg morphine (Pelloux et al. 2006); however, only low novelty seeking rats showed significant place preference at 1.25 mg/kg morphine. Taken together, the present findings suggest that response to novelty may help to predict, in adolescents, the locomotor stimulating effects of morphine, but not its rewarding effects.
Acknowledgements
The authors thank Jason Persyn, Chris Limas, Bindumahi Sudaabattula, and Sonia Cano for technical assistance. The work was supported by the US Public Health Service Grant DA23261
References
- Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference in the developing rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1983. p. 54. [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Dickinson SD, Grahame NJ, Okorn DM, McMullin CS. Genetic differences in cocaine-induced conditioned place preference in mice depend on conditioning trial duration. Psychopharmacology. 1999;146:73–80. doi: 10.1007/s002130051090. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology. 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:10671080. [PubMed] [Google Scholar]
- Doherty JM, Frantz KJ. Heroin self-administration and reinstatement of heroin-seeking in adolescent vs. adult male rats. Psychopharmacology. 2012;219:763–773. doi: 10.1007/s00213-011-2398-x. [DOI] [PubMed] [Google Scholar]
- Doherty JM, Frantz KJ. Attenuated effects of experimenter-administered heroin in adolescent vs. adult male rats: physical withdrawal and locomotor sensitization. Psychopharmacology. 2013;225:595–604. doi: 10.1007/s00213-012-2847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J, Ogbomnwan Y, Williams B, Frantz K. Age-dependent morphine intake and cue-induced reinstatement, but not escalation in intake, by adolescent and adult male rats. Pharmacol Biochem Behav. 2009;92:164–172. doi: 10.1016/j.pbb.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Wellman PJ, Eitan S. Different affective response to opioid withdrawal in adolescent and adult mice. Life Sci. 2009;84:52–60. doi: 10.1016/j.lfs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature. 2005;438:854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- Ingram SL, Fossum EN, Morgan MM. Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacol. 2007;32:600–606. doi: 10.1038/sj.npp.1301139. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Shibanoki S, McGaugh JL. Direct correlation between level of morphine and its biochemical effect on monoamine systems in mouse brain. Biochem Pharmacol. 1983;32:1473–1478. doi: 10.1016/0006-2952(83)90468-9. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EM, Iversen SD. The effect of morphine applied locally to mesencephalic dopamine cell bodies on spontaneous motor activity in the rat. Neurosci Lett. 1979;14:207–212. doi: 10.1016/0304-3940(79)96149-4. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Sensitization to repeated morphine injection in the rat: possible involvement of A10 dopamine neurons. J Pharmacol Exp Ther. 1987;241:204–212. [PubMed] [Google Scholar]
- Koek W. Effects of repeated exposure to morphine in adolescent and adult mice: age-dependent differences in locomotor stimulation, sensitization, and withdrawal. Psychopharmacology. 2013 doi: 10.1007/s00213-013-3298-z. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, France CP, Javors MA. Morphine-induced motor stimulation, motor incoordination, and hypothermia in adolescent and adult mice. Psychopharmacology. 2012;219:1027–1037. doi: 10.1007/s00213-011-2432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H. Effects of interdose interval on ambulatory sensitization to methamphetamine, cocaine and morphine in mice. Eur J Pharmacol. 1996;316:1–5. doi: 10.1016/s0014-2999(96)00635-8. [DOI] [PubMed] [Google Scholar]
- Luo J, Jing L, Qin W-J, Zhang M, Lawrence AJ, Chen F, Liang J-H. Transcription and protein synthesis inhibitors reduce the induction of behavioural sensitization to a single morphine exposure and regulate Hsp70 expression in the mouse nucleus accumbens. Int J Neuropsychopharmacol. 2011;14:107–121. doi: 10.1017/S146114571000057X. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol. 2012;108:1620–1630. doi: 10.1152/jn.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62:851–869. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y. Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacol Biochem Behav. 2013;110:112–116. doi: 10.1016/j.pbb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Costentin J, Duterte-Boucher D. Novelty preference predicts place conditioning to morphine and its oral consumption in rats. Pharmacol Biochem Behav. 2006;84:43–50. doi: 10.1016/j.pbb.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Phillips AG, LePiane FG. Reinforcing effects of morphine microinjection into the ventral tegmental area. Pharmacol Biochem Behav. 1980;12:965–968. doi: 10.1016/0091-3057(80)90460-8. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Quertemont E, Brabant C, Tirelli E. Response to novelty as a predictor for drug effects: the pitfalls of some correlational studies. Psychopharmacology. 2004;173:221–224. doi: 10.1007/s00213-004-1796-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology (Berl) 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder Ch, LeFevour A. Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. Eur J Pharmacol. 1996;299:33–39. doi: 10.1016/0014-2999(95)00852-7. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Thompson AC. Sensitization to the conditioned rewarding effects of morphine and cocaine: differential effects of the kappa-opioid receptor agonist U69593. Eur J Pharmacol. 1998;345:27–34. doi: 10.1016/s0014-2999(97)01614-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Horowitz GP, Lipovsky J. Altered behavioral responsivity to morphine during the periadolescent period in rats. Behav Brain Res. 1982;4:279–288. doi: 10.1016/0166-4328(82)90005-5. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Substance Abuse and Mental Health Services Administration. Rockville MD: Office of Applied Studies; 2003. Overview of Findings from the 2002 National Survey on Drug Use and Health. [Google Scholar]
- Talbot JN, Happe HK, Murrin LC. Mu opioid receptor coupling to Gi/o proteins increases during postnatal development in rat brain. J Pharmacol Exp Ther. 2005;314:596–602. doi: 10.1124/jpet.104.082156. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effetcs, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Reassessment of buprenorphine in conditioned place preference: temporal and pharmacological considerations. Psychopharmacology. 2004;172:58–67. doi: 10.1007/s00213-003-1626-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Herve D, Girault J-A. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacol. 2010;35:401–415. doi: 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, De Vries TJ, Wardeh G, Hogenboom FA, Schoffelmeer AN. A single exposure to morphine induces long-lasting behavioural and neurochemical sensitization in rats. Eur J Neurosci. 2001;14:1533–1538. doi: 10.1046/j.0953-816x.2001.01775.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Curr Top Behav Neurosci. 2010;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Tjon GH, Nestby P, Mulder AH, Schoffelmeer AN, De Vries TJ. Morphine-induced long-term sensitization to the locomotor effects of morphine and amphetamine depends on the temporal pattern of the pretreatment regimen. Psychopharmacology. 1997;131:115–122. doi: 10.1007/s002130050273. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Morphine conditioned place preference and locomotion: the effect of confinement during training. Psychopharmacology. 1987;93:257–260. doi: 10.1007/BF00179944. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DA, Holtzman SG. Periadolescent morphine exposure alters subsequent behavioral sensitivity to morphine in adult rats. Eur J Pharmacol. 2005;528:119–123. doi: 10.1016/j.ejphar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- White DA, Michaels CC, Holtzman SG. Periadolescent male but not female rats have higher motor activity in response to morphine than do adult rats. Pharmacol Biochem Behav. 2008;89:188–199. doi: 10.1016/j.pbb.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology. 2009;34:912–922. doi: 10.1038/npp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]