Abstract
During post-analytical phase, critical value notification to responsible caregiver in a timely manner has potential to improve patient safety which requires cooperative efforts between laboratory personnel and caregivers. It is widely accepted by hospital accreditors that ineffective notification can lead to diagnostic errors that potentially harm patients and are preventable. The objective of the study was to assess the variables affecting critical value notification, their role in affecting it’s quality and approaches to improve it. In the present study 1,187 critical values were analysed in the Clinical Chemistry Laboratory catering to tertiary care hospital for neuropsychiatric diseases. During 25 months of study period, we evaluated critical value notification with respect to clinical care area, caregiver to whom it was notified and timeliness of notification. During the study period (25 months), the laboratory obtained 1,279 critical values in clinical chemistry. The analytes most commonly notified were sodium and potassium (20.97 & 20.8 % of total critical results). Analysis of critical value notification versus area of care showed that critical value notification was high in ICU and emergency area followed by inpatients and 64.61 % critical values were notified between 30 and 120 min after receiving the samples. It was found that failure to notify the responsible caregiver in timely manner represent an important patient safety issue and may lead to diagnostic errors. The major area of concern are notification of critical value for outpatient samples, incompleteness of test requisition forms regarding illegible writing, lack of information of treating physician and location of test ordering and difficulty in contacting the responsible caregiver.
Keywords: Critical value notification, Alert value, Panic value, Turnaround time, LIS
Introduction
A quality reporting system is defined as the delivery of correct results to the appropriate clinicians in a time frame that ensures patient safety without overburdening both the clinicians and laboratory team [1]. Critical value notification is widely accepted in the diagnostic fraternity as an important factor which may affect the patient care and safety. In order to improve this process, the JCAHO has made the “critical value notification”, a National Patient Safety Goal for the years 2004 through 2006 [2]. As per Joint Commission, the 2nd goal states that laboratories need to improve both the timeliness of reporting of critical values and notifying the responsible caregiver of the same [3]. Other accreditation bodies like NABL has technical requirement for reporting critical values in clause 5.8 of 15,189:2012 which is most widely accepted standard by medical laboratory community [4]. College of American Pathologists (CAP) and other international accreditation bodies support improvements in the communication of critical values [5]. Since 2001, the CAP’s Q-Probes program has been used as an excellent tool for comparison of data among laboratory which allows revisions of critical results and values [6, 7].
However, it can be very problematic in small laboratories due to the absence of consensus in laboratory community in selection of critical cut off and formulation of a standard list or target time frames for reporting of critical results. It is important to include only those tests and their cut off in critical list which meet the criterion of the ‘imminent danger’ as expansion of critical call back lists may dilute the urgency of the critical value call [8]. The list of critical value notification should be reviewed periodically to reduce the number of calls made by the technicians and avoid unnecessary interruption for clinicians. In addition to this, for critical values notification to be effective, there are number variables affecting it which must be understood and addressed by the laboratories. Hence, the present study was developed by the laboratory with the aims to improve the system for rapid notification by decreasing the rate of unsuccessful call back i.e. calls that are abandoned or call back made outside the useful time frame and by notification to the concerned care giver (physician, nursing staff). In the present study, we analysed 25 months of critical value data to identify the variables- area of care, timeliness of call back and appropriate caregiver approached and their effect on critical value notification.
Materials and Methods
Study Design
The study was conducted in the Institute of Human Behavior & Allied Sciences, Delhi, a tertiary care institute catering the population of North India for neuropsychiatric disorders. It was initiated in June, 2010 to analyse the variables affecting critical results notification, in the clinical laboratory. The laboratory conducts biochemical testing on all the samples received from the hospital and analyse them for biochemical testing on fully automated Discrete Biochemistry Analyser Olympus AU 480 from Olympus Life Science Research Europa Gmbh. From 1st June, 2010–30th June, 2013, the laboratory performed 478,980 biochemical tests on samples received from inpatients, outpatients, intensive care unit (ICU) and emergencies. During this period 1,187 critical values obtained from clinical chemistry were included and examined in the critical value analysis.
Critical Value Notification
The critical value notification list for biochemistry, in use at our institute, was developed in concurrence with the treating physician as per their requirement for management of patients. The laboratory has a policy to flag each test result requiring critical value notification. The laboratory staff after making call to the care giver, document all the details of phone call including care area, name of the test and it’s value, to whom it was notified and the time at which notification was done. Also, laboratory has a policy of ‘read back’ of critical value by the person who was informed on phone.
The critical results were prospectively identified in the clinical chemistry laboratory and included glucose, electrolytes (sodium, potassium, calcium), creatine phosphokinase and ammonia. For each critical result included in the study, the following variables were recorded: (i) area of care from where patient’s sample was received (Inpatient/ICU & Emergency/Outpatient), (ii) identity of the caregiver who received the result (Physician/Nursing staff/Others including clerk or attendant), (iii) time taken from sample receiving to the time at which critical result was communicated to the caregiver (<30 min/>30 min to <2 h/>2 h).
Data Collection and Analysis
Descriptive statistics was calculated for all the data of variables collected during critical value notification.
Results
Critical Value Notification Versus Area of Care
During the study period, the laboratory obtained 1,279 critical values in clinical chemistry. Out of total critical reports, 1,187 were notified, which constituted 0.25 % of the total test results reported by the laboratory (478,980). The analytes most commonly notified were sodium (249 results; 20.97 % of total critical results) and potassium (247 results; 20.80 % of total critical results) (Table 1). However, critical value notification with respect to total volume of that particular test was maximal for ammonia (125/1,392 ~ 8.97 %) followed by creatine phosphokinase (152/2,304 ~ 6.59 %).
Table 1.
Critical values by test
| Test | Total test volume | Critical test results | Percentage of all critical value notification | Percentage of total test volume with it’s critical value notification |
|---|---|---|---|---|
| Glucose | 29,346 | 183 | 15.41 | 0.62 |
| Sodium | 80,604 | 249 | 20.97 | 0.31 |
| Potassium | 80,604 | 247 | 20.80 | 0.31 |
| Calcium | 80,604 | 231 | 19.46 | 0.29 |
| Creatine phosphokinase | 2,304 | 152 | 12.80 | 6.59 |
| Ammonia | 1,392 | 125 | 10.53 | 8.97 |
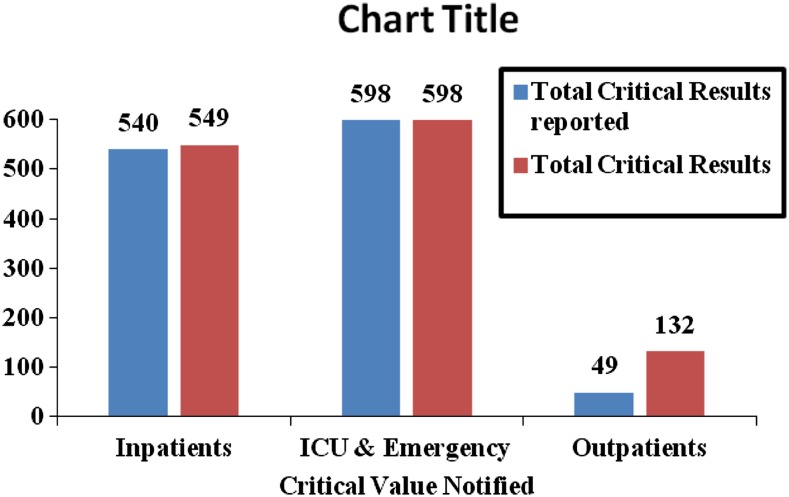
Analysis of critical value notification versus area of care (Fig. 1) showed that critical value notification was high in ICU and emergency area (598 critical values notification) followed by inpatients (540 critical values notification). Thus, ICU & emergencies and inpatient tests were more than 11 times likely to result in a critical value notification than outpatient tests.
Fig. 1.
Total critical values notified (June 2010–June 2013)
Critical Value Notification Versus Care Givers
Present study showed that reaching to the appropriate care giver is a complicated task especially for inpatient and outpatient critical value notification. It is still much more difficult to reach the responsible care giver for outpatient critical value notification as there is no fixed patient location to reach. Also, the critical results may not be communicated to the treating physician but to the nursing staff and others who perform clinical support function. Table 2 shows that only 25.77 % clinicians could be notified about critical value whereas 52.90 % critical values were notified to the nursing staff and rest to others. All the critical value notified to the responsible care giver were recorded in log book which included time at which call was made, patient identifiers (name, age, sex, registration number), the name of the test and it’s result and to whom critical value was notified. Out of all critical values, only 0.02 % (92 reports) critical values could not be notified to the responsible care giver and 83 cases were of outpatients (90.21 %). Hence, outpatient critical value notification is challenging and need attention in developing different approaches to improve the notification.
Table 2.
Critical value notification
| Critical value notification versus area of care | Critical value notification versus time | Critical value notification versus caregivers | ||||||
|---|---|---|---|---|---|---|---|---|
| Inpatients | ICU | Outpatients | <30 min | >30 min and <2 h | >2 h and <6 h | Nursing staff | Others | Consultant/SR/JR |
| 540 | 598 | 49 | 380 | 767 | 40 | 628 | 253 | 306 |
Critical Value Notification Versus Turnaround Time
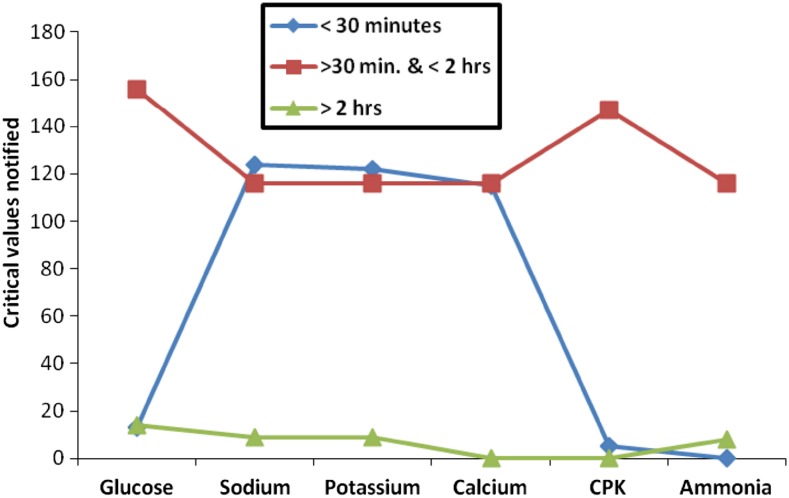
The timeliness of critical value reporting was assessed in the laboratory, by dividing the turnaround time in three slots (i) critical value notified within 30 min of receiving the sample, (ii) critical value notified after 30 min but within 2 h of receiving the sample (iii) critical value notified after 2 h of receiving the sample. It is important to determine the turnaround time for critical value notification by each laboratory individually. Once the specimen is received in the laboratory, time of receiving is noted in the log book. After sample collection, we observed that laboratory takes approximately 30–40 min to detect a critical abnormality. Additional 3–5 min are taken by the laboratory staff on an average to notify clinical findings to the treating physician or nursing staff. As shown in Table 2, it was found in the present study that more than 50 % (64.61 %) critical values were notified between 30 and 120 min after receiving the samples. One third critical reports were communicated to the concerned caregivers within 30 min (32.01 %). Only 40 critical values (0.034 %) were notified after 2 h. Out of all the analytes notified in the present study, 49.79 % sodium, 49.39 % potassium and 49.78 % calcium critical values were notified within 30 min of sample receiving (Fig. 2). There were only 18 instances of delayed notification of electrolytes within study period (June 2010–June 2013). Such delays were related to the testing performed on outpatients and lack of information in test requisition form (TRF) regarding name of the treating physician or location of test ordering. During study period, laboratory received 6.92 % incomplete TRFs.
Fig. 2.
Critical value notification versus turnaround time
Discussion
Critical value notification is an integral part of clinical laboratories by which it participates in patient safety initiatives. As per CLIA definition a test result that indicates an immediately ‘life threatening’ condition is identified as critical value [9]. Alternate terms used when referring to these test results are panic or alert values [9]. In the present study, we provide the details of operational aspects of critical value notification including role of variables like area of care i.e. location of test ordering, care giver approached during notification and timeliness of notification on it. This analysis may help in providing initiatives to laboratories for comparison and process improvement.
In our laboratory, most commonly notified critical results were for sodium, potassium and calcium. The average TAT for critical value notification in our laboratory was 60 min. Our study shows that electrolytes in more than 50 % cases were notified for critical value within 30 min. This could be attributed to many factors. Our laboratory has a written policy and procedures for notification of critical values. As per laid down procedure, a log book is maintained which included name of the patient, age, sex, registration number, location of the patient, the name of the test and it’s result and to whom critical value was notified and at what time. At the end of the month, TAT is calculated for all the critical values notified and for critical values notified beyond TAT, root cause analysis is done to take corrective and preventive action. Another important factor was regular sensitisation of laboratory staff regarding these policies and procedures leading to increased awareness among laboratory staff. However, there were number of instances during study period when notification was delayed beyond TAT. Such delays in critical value notification correlated with inadequate information of patient in TRF like name of the ordering clinician, ordering location, difficulty in communication to the appropriate care giver. Later problem was not faced in critical value notification in inpatients, ICU and emergency as it is much easier to contact nursing staff in these areas due to their fixed location. But communication with treating clinician was a difficult task. Plebani et al. [10] have reported communication as one of the major hurdle in critical value reporting. Outpatient critical value notification was a unique challenge where it was not only difficult to approach the responsible caregiver, but also in timely notification. Lack of fixed location for notification, generation of most of the outpatient reports after OPD hours and missing or illegible information of ordering clinician on TRF led to non reporting or delayed notification of critical value in outpatients. Digh et al. [8] also found delayed reporting of critical values of the specimen being obtained from an outpatient as there is no fixed patient location that can be contacted.
This study highlighted that there are number of approaches to improve the critical value notification and it’s TAT. First, the critical value notification policy must be laid down by the laboratory. There should be a well written policy for critical value notification and should include list of critical values to be notified, person identified from the laboratory to notify, to whom it will be notified, TAT for notification, mode of notification. As per procedure for notification, laboratory should maintain a log book for the same. The established list of critical values must be periodically reviewed, revised and updated in consultation with the clinicians. Long and complex list of critical value must be avoided as that requires significant laboratory man power for notification. It is desirable to have a comprehensive list as increased number of calls may dilute the urgency of critical value call leading to unnecessary interruptions for clinicians [8]. Second, we found that increased laboratory personnel and caregiver’s awareness regarding the laid down policy and procedure of critical value notification led to improvement in communication of critical value and timely notification of it. There is a need for implementation of practices to improve communication between the laboratories and caregivers.
Another solution for improving the process of critical value notification is use of Laboratory Information System (LIS) which automatically detect critical values and communicate with the responsible caregiver for critical value, thereby, reducing the critical value reporting time [11]. However, the automated reporting system must reliably determine not only the identification of person providing the information, but also, the personnel to whom information was communicated, along with the acknowledgement for the same. Such system must also have mechanism of delta check (i.e. change in the current test result from the previous results) [8]. Presently, some clinical laboratories are also providing mobile phones to clinicians which are linked to the information system to provide real time critical value notification to the physician on call. Introduction of such automated communication system avoids the potential errors in communication and improves the rate of successful notification and shortening the notification times. In the laboratory such efforts should be made to prevent diagnostic errors that potentially harm patients and improve the reliability of laboratory for patient care and safety.
Conclusion
The 4 most important findings of this study are (1) critical value notification was highest (50.37 % of total critical value notification) for ICU and emergency area, (2) 52.90 % critical value are notified to the nursing staff, (3) electrolytes—sodium, potassium and calcium have minimal turnaround time for notification as almost 50 % critical values were notified within 30 min whereas laboratory’s average turnaround time is 60 min, (4) only 37.21 % critical values could be notified for outpatients.
Acknowledgments
Conflict of interest
None.
Contributor Information
Rachna Agarwal, Email: rachna1000@hotmail.com.
Neelam Chhillar, Email: nlmchhillar@yahoo.co.in.
Chandra B. Tripathi, Email: cbt_vns@hotmail.com
References
- 1.Piva E, Plebani M. Interpretative reports and critical values. Clin Chim Acta. 2009;404:52–58. doi: 10.1016/j.cca.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 2.Joint Commission on the Accreditation of Healthcare Organisations: National Patient Safety Goals. Available at: http://www.jcaho.org/accredited+organisations/patient+safety/npsg.htm.
- 3.The Joint Commission. National Patient Safety Goals: 2013 National Patient Safety Goals. http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals. Accessed 16 July 2013.
- 4.ISO 15189. Medical laboratories: particular requirements for quality and competence. Geneva: International Organisation for Standardisation; 2012.
- 5.College of American Pathologists. Association and laboratory improvement. http://www.cap.org/apps/cap.portal; 2009.
- 6.Howanitz PJ, Steindel SJ, Heard NV. Laboratory critical values policies and procedures: a College of American Pathologists Q-Probes Study in 623 institutions. Arch Pathol Lab Med. 2002;126:663–669. doi: 10.5858/2002-126-0663-LCVPAP. [DOI] [PubMed] [Google Scholar]
- 7.Elisa P, Sciacovelli L, Laposata M, Plebani M. Assessment of critical values policies in Italian institutions: comparison with the US situation. Clin Chem Lab Med. 2010;48:461–468. doi: 10.1515/CCLM.2010.096. [DOI] [PubMed] [Google Scholar]
- 8.Dighe AS, Rao A, Coakley AB, Lewandrowski KB. Analysis of laboratory critical value reporting at a large academic medical centre. Am J Pathol. 2006;125:758–764. doi: 10.1309/R53X-VC2U-5CH6-TNG8. [DOI] [PubMed] [Google Scholar]
- 9.Wager EA, Friedberg RC, Souers R, Stakovic AK. Critical values comparison. A college of American Pathologists Q-Probes Survey of 163 clinical laboratories. Arch Pathol Lab Med. 2007;131:1769–1775. doi: 10.5858/2007-131-1769-CVCACO. [DOI] [PubMed] [Google Scholar]
- 10.Plibani M, Zaninotto M, Sciacovelli L, Piva E, Saw S. Critical laboratory results: communication is just one of the problems. Am J Clin Pathol. 2012;137:164. doi: 10.1309/AJCPTCJQAO1SV8IJ. [DOI] [PubMed] [Google Scholar]
- 11.Parl F, O leary MF, Kaiser AB, Paulett JM, Statnikova K, Schultz EK. Implementation of closed-loop reporting system for critical values and clinical communication in compliance with goals of the joint commission. Clin Chem. 2010;56(3):417–423. doi: 10.1373/clinchem.2009.135376. [DOI] [PubMed] [Google Scholar]