Abstract
Dofetilide is an effective antiarrhythmic agent for conversion of atrial fibrillation and atrial flutter as well as maintenance of sinus rhythm in appropriately selected patients. However, as with other antiarrhythmic agents, proarrhythmia is a known adverse effect. The risk of dofetilide induced torsade de pointes (Tdp) is low when used with strict dosing criteria guided by renal function, QT interval and concomitant drug therapy. Benefit from dofetilide use must be individualized and weighed against the side effects and the role of other available treatment options. In this review, we discuss the underlying mechanism, risk factors and precautionary measures to avoid dofetilide induced QT prolongation and ventricular tachycardia/Tdp. We suggest a scheme for the management of QT prolongation, ventricular arrhythmia and Tdp as well.
Keywords: Dofetilide, Torsade de Pointes, Proarrhythmia, QT prolongation, Atrial fibrillation
1. Background
The last decade has witnessed a large increase in the prescription of antiarrhythmic drugs (AADs). The use of AADs is predicted to rise due to the increasing incidence of atrial fibrillation in our aging population. Several classes of AADs have been shown to be effective in maintaining sinus rhythm. However, most of these agents are also proarrhythmic and many have been associated with severe and occasionally fatal arrhythmias, most commonly presenting as torsade de pointes (Tdp). Dofetilide is a class III antiarrhythmic drug which is effective in maintaining sinus rhythm, but due to the significant proarrhythmic risk initiation requires hospital monitoring. We describe an update on the mechanism, risk factors, and preventive measures associated with dofetilide induced tachyarrhythmia, and propose an evidence based model on monitoring and management of ventricular arrhythmia/Tdp associated with dofetilide.
2. Dofetilide pharmacodynamics and pharmacokinetics
Dofetilide is almost completely absorbed after oral administration with a bioavailability of more than 90% and reaches peak plasma concentration in approximately 2 h irrespective of the dose. The steady-state plasma concentration is reached by the third day of a twice-daily dosing regimen. Females tend to have 14–22% higher plasma concentration than males even after correcting for body weight and creatinine clearance. After oral administration, plasma dofetilide has a linear relationship with QTc interval. Dofetilide is mainly eliminated (approximately 80%) through the kidneys via passive glomerular filtration and cationic tubular secretion. The remainder is excreted in feces (<10%) or metabolized in liver, predominantly by the cytochrome P4503A4 family, into inactive metabolites. It exhibits first-order elimination kinetics with a half-life of 8–10 h in patients with normal renal function. Patients with severe hepatic impairment have not been studied.
Dofetilide is a pure Vaughan Williams class III antiarrhythmic drug. It acts by blocking the rapid component of the cardiac delayed rectifier K+ current (IKr), which is encoded by human ether-à-go-go–related gene (hERG).1 This results in prolongation of action potential duration and effective refractory period. At the recommended doses, it causes selective prolongation of the QT interval on the surface electrocardiogram (EKG) without any effect on PR, QRS, AH or HV intervals.2 This happens due to the lack of effect on slow component of the delayed rectifier potassium current (Iks), the inward current (IK1), any sodium channel (INa), calcium channel (ICa) or beta-receptor.3 To sum up, dofetilide does not affect resting membrane potential, maximum upstroke velocity of the action potential (Vmax) and conduction velocity, and it has minimal effects upon myocardial contractility and systemic hemodynamics. Dofetilide prolongs the refractory period in the atria to a greater extent than in the ventricle. Dose for dose, the increase in atrial effective refractory period is double that of ventricular effective refractory period. This may account for its greater effectiveness in treating atrial arrhythmias.
3. Clinical utility and adverse reactions
Dofetilide is one of the few antiarrhythmic agents which appear to be safe in patients with myocardial infarction and impaired left ventricular function (LVEF).4 In a study done on small number of patients with paroxysmal atrial fibrillation with preserved LVEF dofetilide was found to be not effective in maintenance of sinus rhythm.5
Dofetilide can cause serious ventricular arrhythmias, primarily Tdp. Most commonly this is seen in patients due to drug interaction or serum electrolyte abnormalities (Tables 1 and 2) which can cause higher serum concentration of dofetilide. However, ventricular tachyarrhythmias have been reported in patients on dofetilide in the absence of precipitating factors.6
Table 1.
Factors associated with increased risk of developing drug-induced excessive QT prolongation and Tdp.
|
|
|
|
|
|
|
|
|
|
Adapted with permission from.17
Table 2.
Dofetilide drug interaction (common drugs).
|
|
|
|
3.1. Torsade de pointes
Tdp is a polymorphic ventricular tachycardia associated with QT interval prolongation which can degenerate into ventricular fibrillation causing sudden cardiac death. A typical mode of onset involves short-long-short sequence of RR intervals (Fig. 2) which is also known as pause dependent phenomenon.7 However, other mechanism of Tdp development has been suggested in patients on beta blockers.7 So, Tdp normally occurs under conditions in which repolarization reserve8 is challenged beyond capacity, as in the long QT syndrome (LQTS), or in congestive heart failure.9
Fig. 2.

Tele strips showing precipitation of episodes of Tdp (Panel A, B, C & D) during continuous telemonitoring with the classic features including a prolonged QT interval (Panel A), initiation of the arrhythmia after a short-long-short cycle sequence by a PVC that falls near the peak of the distorted T wave (Panel B & C), “warm-up” phenomenon with initial R–R cycles longer than subsequent cycles, and abrupt switching of QRS morphology from predominately positive to predominately negative complexes (Panel A & B).
Ventricular repolarization (Fig. 3) is achieved by rapid [IKr] and slow [IKs] delayed rectifying potassium currents. When these currents are reduced, repolarization is prolonged and the QT interval increases. Both early and delayed depolarizing currents, which may ensue during these prolonged repolarization periods, may trigger a ventricular arrhythmia. Tdp is classically associated with early-after depolarizations (EADs). EADs are caused by inward calcium currents10 either due to reopening of L-type calcium channels or sodium channels or from current generated via augmented sodium–calcium exchange. Ectopic beats occur when the amplitude of an EAD reaches a critical threshold and occurs in a large enough region of the heart.11 Usually repolarization defects due to IKs blockage are well tolerated and do not lead to Tdp. This can be explained by potential compensation by other K currents, mainly IKr. Agents which block IKr could induce QT interval prolongation and Tdp. Therefore, conditions and drugs that increase the number of L-type calcium channels in cardiac fiber membranes may facilitate the induction of Tdp in presence of delayed repolarization.
Fig. 3.
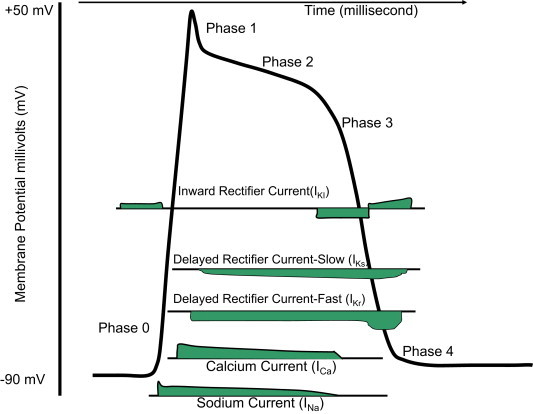
Schematic representation of action potential of a ventricular myocyte. Rapid depolarization (phase 0) is caused by opening of the fast sodium channels resulting a transient increase inward sodium current (INa). Phase 1 characterizes an initial repolarization is a result of a short-lived, hyperpolarizing outward K+ current (IKto) caused by the opening of a special type of transient outward K+ channel (Kto). Phase 2 or the plateau phase/slow repolarization represents delay in the repolarization which caused by the inward calcium movement through long-lasting calcium channels (ICa L) that open up when the membrane potential depolarizes to about −40 mV. Phase 3 or fast repolarization occurs when rectifier potassium channels (IKr and IKs) open with simultaneous inactivation of the calcium channels. Phase 4 represents resting membrane potential.
Drugs are the most common cause of Tdp. Approximately 2–3% of all prescriptions are estimated to involve medications that may unintentionally cause acquired long QT syndrome (LQTS).12 However, the incidence of drug-induced Tdp is relatively low. This is likely a result of individual differences in pharmacokinetics and pharmacogenomics. Other causes include structural heart diseases including ischemia, congestive heart failure, cardiomyopathy; bradycardia, subarachnoid hemorrhage, human immunodeficiency virus (HIV) infection and electrolyte abnormalities. Hypokalemia is associated with decreased conduction velocity, shortening of the effective refractory period, prolongation of the relative refractory period, increased automaticity and EADs.13 Hypomagnesemia causes EADs due to its modulating effect on L-type calcium channels. The proarrhythmic effect of hypomagnesemia is increased in the presence of hypokalemia and bradycardia. Because time for repolarization (the QT interval) is rate dependent, Tdp is more likely to occur during slower heart rates.14 Hypocalcemia has been associated with Tdp only in rare cases.15 In the absence of these factors congenital QT syndrome (LQTS) could be a culprit.
It has been proposed that subjects susceptible to drug-induced Tdp may have underlying genetic mutations, which would become apparent when exposed to a QT-prolonging drug. Further, it has been speculated that some cases of drug-induced LQTS might be associated with silent mutations and common polymorphism in genes responsible for the congenital LQTS, such as KCNQ1 encoding slowly activating delayed rectifier potassium currents (IKs). However, it remains unclear why subclinical IKs dysfunction is a risk of drug-LQTS.16
4. Risk prediction from electrocardiogram (EKG) assessment
Surface EKG can predict Tdp risk in majority of patients. There are several EKG findings which may be associated with increased risk (Fig. 1). In majority of cases, the development of Tdp is inevitably accompanied by QT interval prolongation. Tdp has also been seen in the absence of QT prolongation and vice-versa. Usually, this is evident in each heartbeat, but sometimes it occurs only in the beat or two before the Tdp.11 QT interval varies with heart rate and QTc describes a heart rate corrected QT interval. QTc refers to the QT interval heart rate correction, when not otherwise stated, generally refers to the Bazett correction. QTc values greater than 450 ms for men and 470 ms for women are usually considered prolonged QT intervals.17 The Bazett correction tends to produce overlong QTc values at faster heart rates, particularly above 85 beats per minute.15 Alternative QTc calculation methods are available that adjust more appropriately at faster rates.
Fig. 1.
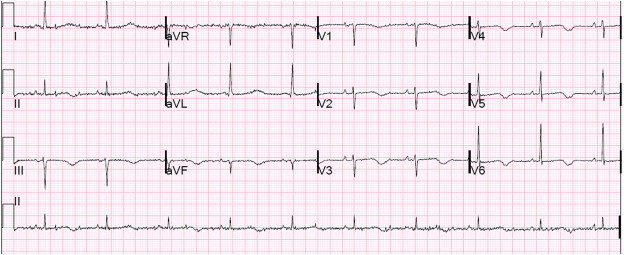
ECG showing prolonged QTc interval at 500 ms.
QT interval is measured from the beginning of the QRS complex to the end of the T Wave. Although the onset of the QRS complex is usually well defined, the end of the T wave is often obscure, especially when T waves are of low amplitude or T wave–U wave distortion is present. In a 12-lead ECG, the lead with well defined T wave ending and amplitude of at least 2 mm should be used to measure QT interval. In cases where the end of the T wave is difficult to determine (e.g., biphasic or notched T waves, T waves with superimposed U waves), it can be determined by drawing a line from the peak of the T wave following the steepest T-wave down slope. The intersection of this line with the isoelectric baseline is considered the end of the T wave.15
Calculation of QTc in the setting of atrial fibrillation could be challenging due to beat to beat variability of the RR intervals. One way to deal with the irregularity of the rhythm is to identify the shortest and longest R–R intervals, calculate the QTc for each, and mean of these QT intervals may represent QTc.15 Alternatively, a rhythm strip can be used determine whether, on average, the interval from R wave to the peak (or nadir) of the T wave is more than 50% of the R–R interval. A wide QRS complex will increase the total QT interval. Such an increase of the QT interval due to a new conduction block should not be considered indicative of acquired LQTS and risk for Tdp. QTc should be measured on 12-lead EKG using standard equipment in a consistent manner for a given patient.
A normal QT at baseline does not preclude excessive QT prolongation and even development of Tdp when exposed to a QT-prolonging drug.18 In the absence of marked QT prolongation, Tdp risk could be explained by QT dispersion.17 QT dispersion, defined as the inter-lead variation of QT duration in a surface EKG. QT dispersion is calculated from the difference between the maximum and the minimum QTc in precordial leads. Values greater than 80 ms are considered abnormally prolonged.
Other EKG features which may be associated with increased risk of Tdp include interval from peak to the end of the T wave, presence of T-wave alternans (change in amplitude or polarity of T wave on alternating beats) and “T–U waves”.19 Clinically, the EAD risk correlates well with the presence of T wave humps, T-U waves or bifid T waves that often precede the development of Tdp.20 However, exaggerated QT interval prolongation with T-U distortion after a pause should be considered a strong marker of risk for Tdp.
4.1. Dofetilide-induced Tdp
Among class III antiarrhythmics, sotalol and dofetilide are associated with higher risk of Tdp than amiodarone despite the fact that all of these agents can cause QT prolongation and bradycardia. It has been suggested that dofetilide can induce Tdp by multiple mechanisms including prolongation of repolarization interval, slowing of heart rate, and increasing repolarization heterogeneities in the ventricular myocardium (manifested by increased QT dispersion in surface ECG). However, for patients with congestion and reduced left ventricular systolic function, changes in QT dispersion following treatment with dofetilide do not predict cardiac mortality.21
Dofetilide exhibits reverse use dependence, which means the duration of the action potential increases at slow heart rates, increasing the risk for EADs and, therefore, Tdp. As discussed earlier higher risk of Tdp is seen in patients who have factors (Table 1) which cause prolonged QT interval and or which facilitate formation of EAD. Torp-Pedersen et al studied the risk factors of Tdp in patients with left ventricular systolic dysfunction either due to heart failure (HF) or recent myocardial infarction (MI) treated with dofetilide. They concluded that severity of HF (Class III or IV, odd ratio – 3.9), female gender (Odds ratio – 3.2), and baseline QTc duration pose higher risk of early Tdp when treated with dofetilide.22 It has been proposed that women have a reduced cardiac ‘repolarization reserve’ resulting in a longer baseline-corrected QT interval possibly due to influence of estrogen.23 This is supported by the higher risk of cardiac events in prepubertal boys and higher in women during adulthood.24 Data from the International LQTS Registry are consistent with this hormonal theory.
In studies of patients with supraventricular arrhythmias, ventricular tachycardia occurred in 2.0% and Tdp was seen in 0.8% of the patients.25 In The Danish Investigations of Arrhythmia and Mortality on Dofetilide (DIAMOND) studies,22 the majority of Tdp and cardiac arrest occurred within the first three days when the patient was in a monitored hospital setting. Before 1994, Dofetilide dosing was not adjusted for renal dysfunction and clinical trials used doses upto 750 μg. This led to higher Tdp rates (upto 4.8%). DIAMOND studies used 500 μg oral twice/day dose and showed a decreased Tdp rate of 1.7%. The incidence of Tdp was 3.3% in the congestive heart failure patients and 0.9% in the patients with a recent myocardial infarction, with respectively 76% and 57% occurring in the first 3 days of initiation of therapy. On the review of published literature in pubmed we estimated a Tdp rate of 2.4% (89/3719) and 7 case reports with a sudden death rate of 0.05% (2/3719) amongst patients who were prescribed dofetilide (Table 3).
Table 3.
Published reports on Torsade de Pointes and sudden death.
| Study | Tdp rate | Arrhythmic death | Study | Tdp rate | Arrhythmic death |
|---|---|---|---|---|---|
| Echt et al, 199533 | 1/18 (5.5%) | Nagra et al, 200526 | 1, case report | ||
| Frost et al, 199734 | 0/65 (0%) | Reiffel, 200535 | 1, case report | ||
| Ventricular tachycardia studies36 | 11/443 (2.4%) | Aktas et al, 200737 | 4, case reports | ||
| DIAMOND CHF, 199922 | 25/762 (3.3%) | Mykytsey et al, 20075 | 3/34 | ||
| DIAMOND MI, 20004 | 7/749 (0.9%) | Kolb et al, 20086 | 1, case report | ||
| SAFIRE-D, 200026 | 2/250 (0.8%) | 1/250 (0.4%) | Banchs et al, 200838 | 2/80 (2.5%) | |
| Bianconi et al, 200039 | 4/48 (8%) | Coleman et al, 200940 | 1/160 (0.6%) | ||
| DIAMOND AF, 200141 | 4/250 (1.6%) | Shamiss et al, 200929 | 0/127 | ||
| Mazur et al, 200142 | 15/87 (17%) | Wells et al, 200943 | 2/30 (6.7%) | ||
| Prystowsky et al, 200344 | 2/69 (2.9%) | 1/69 (1.4%) | Pinter et al, 201145 | 0/18 | |
| Guanzon et al, 200446 | 0/107 | Baquero et al, 201247 | 2/30 (6.7%) potentially? | ||
| Sun et al, 200448 | 7/105 (6.7%) | Manocha et al, 201249 | 1/287 (0.3%) | ||
| Total Tdp Sudden death |
89/3719 (2.4%), and 7 case reports 2/3719 (0.05%) |
||||
4.2. Prevention strategies
Prevention of adverse reactions involves appropriate patient selection and proper monitoring. In the absence of any other contraindication (Tables 1 and 2) dofetilide should be initiated in a hospital setting with continuous cardiac monitoring for the first 3 days, or for a minimum of 12 h after cardioversion, whichever is later. Cardioversion of atrial fibrillation is known to increase the risk of QT prolongation.17 This is due to the fact that dofetilide induces more QT prolongation after rhythm conversion than during rapid atrial fibrillation. In the setting of acute myocardial infarction and atrial fibrillation, myocardial ischemia does not affect Dofetilide induced Tdp. However, a case of Tdp in the setting of MI in an elderly patient has been reported.26 Based on observations made in SAFIRE-D27 and DIAMOND studies an algorithm for the initiation, monitoring and dose adjustments for patients was developed by the manufacturer of dofetilide (Pfizer). It should be avoided in patients with congestive HF requiring aggressive diuresis as diuretics may cause hypokalemia induced EAD and Tdp. Similarly, patients with left ventricular hypertrophy have higher chances of developing EAD and greater degree of QT dispersion or heterogeneous ventricular myocardial repolarization.
Dofetilide should not be used with any other antiarrhythmic agents. It should be only started after 3 half lives of other agent have passed. Due to unpredictable half-life of amiodarone, it should only be started after 3 months or when serum amiodarone level falls below 0.3 μg/ml.28 However, dofetilide has been used in patients after discontinuing Amiodarone for 4–6 weeks without any adverse effects.29
It is recommended that serum creatinine should be checked every three months in patients who are using Dofetilide.28 Subsequent administration of drugs with potential to cause QT prolongation should be avoided in the patients with prior history of drug-induced severe QT prolongation and Tdp. As discussed earlier, women with any other risk factors (Table 1) are at particularly high risk for developing arrhythmia from antiarrhythmics, and should be avoided. Patients with history of sustained ventricular tachyarrhythmias should not be started on such agents.14 In obese patients, dofetilide dosing recommendation is not clear but based on data from studies we recommend that it should be avoided in patients with actual body weight exceeding 40% above ideal body weight.30 We propose a more practical algorithm (Fig. 4) for dofetilide dosing and monitoring which includes recommendations by the manufacturer, SAFIRE-D and DIAMOND studies, and our suggestions.
Fig. 4.
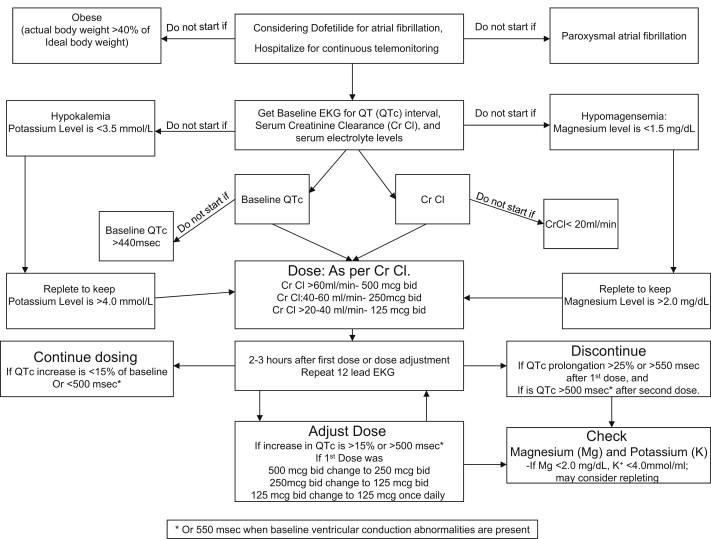
Algorithm for starting dofetilide in a patient with atrial fibrillation.
5. Management of dofetilide induced QT prolongation and Tdp
QT prolongation should lead to prompt withdrawal of the offending drug. An external defibrillator should be made available. Careful assessment of potentially aggravating drug–drug interactions, bradyarrhythmias, or electrolyte abnormalities should be done. We suggest that prophylactic magnesium in cases of severe QT prolongation (>500 ms) after the first dose might decrease the risk of ventricular arrhythmias. Intravenous magnesium reduces the QTc interval of patients receiving ibutilide and oral magnesium lactate lowers the QTc interval of patients receiving sotalol or dofetilide.31
Treatment of dofetilide induced ventricular tachycardia/Tdp is not well described. It should be managed as Tdp from any other cause. Immediate direct-current cardioversion should be performed for patients with Tdp that does not terminate spontaneously or that degenerates into ventricular fibrillation. Even in the absence of hypomagnesemia, intravenous magnesium sulfate should be administered in patients with frequent ventricular arrhythmia and Tdp. Magnesium sulfate 2.0 g can be infused intravenously, and if episodes of Tdp persist, it might be necessary to repeat infusions of magnesium sulfate.32 Repletion of potassium to maintain the serum levels of 4.5 mmol/l to 5 mmol/l may also be considered, although there is little evidence to support this practice.32 If patient's heart rate is on the lower side (<85 beats per minute), isoproterenol or temporary pacing may be used to increase heart rate and hence, shorten QT interval. However, it may not be helpful in every patient as it has been shown that beta-adrenergic stimulation could be arrhythmogenic in conditions of acquired LQTS when subclinical IKs dysfunction is present and heart rate is not fully increased.16 Clinically such distinction between patients cannot be made and we recommend using isoproterenol in Tdp patients if their heart rate is slow.
6. Conclusion
Dofetilide is an effective and relatively safe AAD for conversion to sinus rhythm and maintenance of it in patients with atrial fibrillation. However, caution should be exercised in selecting appropriate patients before starting it as majority of the dofetilide induced proarrhythmic events happen in the presence of another offending factor. Risk factors for dofetilide associated Tdp include older age, female sex, heart disease, electrolyte disorders (especially hypokalemia and hypomagnesemia), renal or hepatic dysfunction, bradycardia or rhythms with long pauses, treatment with other QT-prolonging drugs, and genetic predisposition. The risk-benefit ratio should be assessed for each individual to determine whether the potential therapeutic benefit of the drug outweighs the risk for Tdp. After initiation of a drug associated with Tdp, ECG signs indicative of arrhythmia risk include an increase in QTc from baseline of more than 50 ms, marked QTc interval prolongation >500 ms, T–U wave distortion that becomes more exaggerated in the beat after a pause, visible (macroscopic) T-wave alternans, new-onset ventricular ectopy, couplets and non sustained polymorphic ventricular tachycardia initiated in the beat after a pause. Monitoring of QT intervals before and after drug administration should be done in a consistent method utilizing the same recording device, ECG lead, measurement tool (automated or manual), and heart rate correction formula. Recommended actions when ECG signs of impending Tdp develop should include stopping further doses of the offending drug, replacing potassium, administering magnesium, temporary pacing can be considered to prevent bradycardia (heart rate <75 beats per minute) and long pauses, and transfer the patient to a coronary care unit with continuous telemonitoring with an immediate defibrillation option is available.
Disclosure
Both Abhishek Jaiswal and Seth Goldbarg are currently receiving research grant from Medtronic Inc.
Conflicts of interest
All authors have none to declare.
References
- 1.Redfern W.S., Carlsson L., Davis A.S. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003;58:32–45. doi: 10.1016/s0008-6363(02)00846-5. [DOI] [PubMed] [Google Scholar]
- 2.Sedgwick M.L., Rasmussen H.S., Cobbe S.M. Clinical and electrophysiologic effects of intravenous dofetilide (UK-68,798), a new class III antiarrhythmic drug, in patients with angina pectoris. Am J Cardiol. 1992;69:513–517. doi: 10.1016/0002-9149(92)90996-c. [DOI] [PubMed] [Google Scholar]
- 3.Roukoz H., Saliba W. Dofetilide: a new class III antiarrhythmic agent. Expert Rev Cardiovasc Ther. 2007;5:9–19. doi: 10.1586/14779072.5.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Kober L., Bloch Thomsen P.E., Moller M. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Lancet. 2000;356:2052–2058. doi: 10.1016/s0140-6736(00)03402-4. [DOI] [PubMed] [Google Scholar]
- 5.Mykytsey A., Bauman J.L., Razminia M. Observations on the safety and effectiveness of dofetilide in patients with paroxysmal atrial fibrillation and normal left ventricular function. J Cardiovasc Pharmacol Ther. 2007;12:36–43. doi: 10.1177/1074248407299272. [DOI] [PubMed] [Google Scholar]
- 6.Kolb C., Ndrepepa G., Zrenner B. Late dofetilide-associated life-threatening proarrhythmia. Int J Cardiol. 2008;127:e54–e56. doi: 10.1016/j.ijcard.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Noda T., Shimizu W., Satomi K. Classification and mechanism of Torsade de Pointes initiation in patients with congenital long QT syndrome. Eur Heart J. 2004;25:2149–2154. doi: 10.1016/j.ehj.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Roden D.M. Taking the “idio” out of “idiosyncratic”: predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21:1029–1034. doi: 10.1111/j.1540-8159.1998.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 9.Tomaselli G.F., Beuckelmann D.J., Calkins H.G. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994;90:2534–2539. doi: 10.1161/01.cir.90.5.2534. [DOI] [PubMed] [Google Scholar]
- 10.Choi B.R., Burton F., Salama G. Cytosolic Ca2+ triggers early after depolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome. J Physiol. 2002;543:615–631. doi: 10.1113/jphysiol.2002.024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kannankeril P., Roden D.M., Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010;62:760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Recanatini M., Poluzzi E., Masetti M. QT prolongation through hERG K(+) channel blockade: current knowledge and strategies for the early prediction during drug development. Med Res Rev. 2005;25:133–166. doi: 10.1002/med.20019. [DOI] [PubMed] [Google Scholar]
- 13.El-Sherif N., Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J. 2011;18:233–245. [PubMed] [Google Scholar]
- 14.Wolbrette D.L. Risk of proarrhythmia with class III antiarrhythmic agents: sex-based differences and other issues. Am J Cardiol. 2003;91:39D–44D. doi: 10.1016/s0002-9149(02)03378-7. [DOI] [PubMed] [Google Scholar]
- 15.Drew B.J., Ackerman M.J., Funk M. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–947. doi: 10.1016/j.jacc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aiba T., Shimizu W., Inagaki M. Cellular and ionic mechanism for drug-induced long QT syndrome and effectiveness of verapamil. J Am Coll Cardiol. 2005;45:300–307. doi: 10.1016/j.jacc.2004.09.069. [DOI] [PubMed] [Google Scholar]
- 17.Cubeddu L.X. Iatrogenic QT abnormalities and fatal arrhythmias: mechanisms and clinical significance. Curr Cardiol Rev. 2009;5:166–176. doi: 10.2174/157340309788970397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaab S., Hinterseer M., Nabauer M. Sotalol testing unmasks altered repolarization in patients with suspected acquired long-QT-syndrome–a case-control pilot study using i.v. sotalol. Eur Heart J. 2003;24:649–657. doi: 10.1016/s0195-668x(02)00806-0. [DOI] [PubMed] [Google Scholar]
- 19.Narayan S.M. T-wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol. 2006;47:269–281. doi: 10.1016/j.jacc.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 20.Gbadebo T.D., Trimble R.W., Khoo M.S. Calmodulin inhibitor W-7 unmasks a novel electrocardiographic parameter that predicts initiation of torsade de pointes. Circulation. 2002;105:770–774. doi: 10.1161/hc0602.103724. [DOI] [PubMed] [Google Scholar]
- 21.Brendorp B., Elming H., Jun L. Effect of dofetilide on QT dispersion and the prognostic implications of changes in QT dispersion for patients with congestive heart failure. Eur J Heart Fail. 2002;4:201–206. doi: 10.1016/s1388-9842(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 22.Torp-Pedersen C., Moller M., Bloch-Thomsen P.E. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–865. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 23.Drici M.D., Clement N. Is gender a risk factor for adverse drug reactions? The example of drug-induced long QT syndrome. Drug Saf. 2001;24:575–585. doi: 10.2165/00002018-200124080-00002. [DOI] [PubMed] [Google Scholar]
- 24.Locati E.H., Zareba W., Moss A.J. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the International LQTS Registry. Circulation. 1998;97:2237–2244. doi: 10.1161/01.cir.97.22.2237. [DOI] [PubMed] [Google Scholar]
- 25.Pritchett E.L., Wilkinson W.E. Effect of dofetilide on survival in patients with supraventricular arrhythmias. Am Heart J. 1999;138:994–997. doi: 10.1016/s0002-8703(99)70029-3. [DOI] [PubMed] [Google Scholar]
- 26.Nagra B.S., Ledley G.S., Kantharia B.K. Marked QT prolongation and torsades de pointes secondary to acute ischemia in an elderly man taking dofetilide for atrial fibrillation: a cautionary tale. J Cardiovasc Pharmacol Ther. 2005;10:191–195. doi: 10.1177/107424840501000307. [DOI] [PubMed] [Google Scholar]
- 27.Singh S., Zoble R.G., Yellen L. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE-D) study. Circulation. 2000;102:2385–2390. doi: 10.1161/01.cir.102.19.2385. [DOI] [PubMed] [Google Scholar]
- 28.Elming H., Brendorp B., Pedersen O.D. Dofetilide: a new drug to control cardiac arrhythmia. Expert Opin Pharmacother. 2003;4:973–985. doi: 10.1517/14656566.4.6.973. [DOI] [PubMed] [Google Scholar]
- 29.Shamiss Y., Khaykin Y., Oosthuizen R. Dofetilide is safe and effective in preventing atrial fibrillation recurrences in patients accepted for catheter ablation. Europace. 2009;11:1448–1455. doi: 10.1093/europace/eup293. [DOI] [PubMed] [Google Scholar]
- 30.Lenz T.L., Hilleman D.E. Dofetilide, a new class III antiarrhythmic agent. Pharmacotherapy. 2000;20:776–786. doi: 10.1592/phco.20.9.776.35208. [DOI] [PubMed] [Google Scholar]
- 31.McBride B.F., Min B., Kluger J. An evaluation of the impact of oral magnesium lactate on the corrected QT interval of patients receiving sotalol or dofetilide to prevent atrial or ventricular tachyarrhythmia recurrence. Ann Noninvasive Electrocardiol. 2006;11:163–169. doi: 10.1111/j.1542-474X.2006.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zipes D.P., Camm A.J., Borggrefe M. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death) J Am Coll Cardiol. 2006;48:e247–e346. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 33.Echt D.S., Lee J.T., Murray K.T. A randomized, double-blind, placebo-controlled, dose-ranging study of dofetilide in patients with inducible sustained ventricular tachyarrhythmias. J Cardiovasc Electrophysiol. 1995;6:687–699. doi: 10.1111/j.1540-8167.1995.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 34.Frost L., Mortensen P.E., Tingleff J. Efficacy and safety of dofetilide, a new class III antiarrhythmic agent, in acute termination of atrial fibrillation or flutter after coronary artery bypass surgery. Dofetilide Post-CABG Study Group. Int J Cardiol. 1997;58:135–140. doi: 10.1016/s0167-5273(96)02856-2. [DOI] [PubMed] [Google Scholar]
- 35.Reiffel J.A. A typical proarrhythmia with dofetilide: monomorphic VT and exercise-induced torsade de pointes. Pacing Clin Electrophysiol. 2005;28:877–879. doi: 10.1111/j.1540-8159.2005.00179.x. [DOI] [PubMed] [Google Scholar]
- 36.Mounsey J.P., DiMarco J.P. Cardiovascular drugs. Dofetilide. Circulation. 2000;102:2665–2670. doi: 10.1161/01.cir.102.21.2665. [DOI] [PubMed] [Google Scholar]
- 37.Aktas M.K., Shah A.H., Akiyama T. Dofetilide-induced long QT and torsades de pointes. Ann Noninvasive Electrocardiol. 2007;12:197–202. doi: 10.1111/j.1542-474X.2007.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banchs J.E., Wolbrette D.L., Samii S.M. Efficacy and safety of dofetilide in patients with atrial fibrillation and atrial flutter. J Interv Card Electrophysiol. 2008;23:111–115. doi: 10.1007/s10840-008-9290-6. [DOI] [PubMed] [Google Scholar]
- 39.Bianconi L., Castro A., Dinelli M. Comparison of intravenously administered dofetilide versus amiodarone in the acute termination of atrial fibrillation and flutter. A multicentre, randomized, double-blind, placebo-controlled study. Eur Heart J. 2000;21:1265–1273. doi: 10.1053/euhj.1999.2039. [DOI] [PubMed] [Google Scholar]
- 40.Coleman C.I., Sood N., Chawla D. Intravenous magnesium sulfate enhances the ability of dofetilide to successfully cardiovert atrial fibrillation or flutter: results of the Dofetilide and Intravenous Magnesium Evaluation. Europace. 2009;11:892–895. doi: 10.1093/europace/eup084. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen O.D., Bagger H., Keller N. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001;104:292–296. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- 42.Mazur A., Anderson M.E., Bonney S. Pause-dependent polymorphic ventricular tachycardia during long-term treatment with dofetilide: a placebo-controlled, implantable cardioverter-defibrillator-based evaluation. J Am Coll Cardiol. 2001;37:1100–1105. doi: 10.1016/s0735-1097(01)01106-8. [DOI] [PubMed] [Google Scholar]
- 43.Wells R., Khairy P., Harris L. Dofetilide for atrial arrhythmias in congenital heart disease: a multicenter study. Pacing Clin Electrophysiol. 2009;32:1313–1318. doi: 10.1111/j.1540-8159.2009.02479.x. [DOI] [PubMed] [Google Scholar]
- 44.Prystowsky E.N., Freeland S., Branyas N.A. Clinical experience with dofetilide in the treatment of patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:S287–S290. doi: 10.1046/j.1540-8167.2003.90402.x. [DOI] [PubMed] [Google Scholar]
- 45.Pinter A., Akhtari S., O'Connell T. Efficacy and safety of dofetilide in the treatment of frequent ventricular tachyarrhythmias after amiodarone intolerance or failure. J Am Coll Cardiol. 2011;57:380–381. doi: 10.1016/j.jacc.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 46.Guanzon A.V., Crouch M.A. Phase IV trial evaluating the effectiveness and safety of dofetilide. Ann Pharmacother. 2004;38:1142–1147. doi: 10.1345/aph.1D465. [DOI] [PubMed] [Google Scholar]
- 47.Baquero G.A., Banchs J.E., Depalma S. Dofetilide reduces the frequency of ventricular arrhythmias and implantable cardioverter defibrillator therapies. J Cardiovasc Electrophysiol. 2012;23:296–301. doi: 10.1111/j.1540-8167.2011.02183.x. [DOI] [PubMed] [Google Scholar]
- 48.Sun Z., Milos P.M., Thompson J.F. Role of a KCNH2 polymorphism (R1047 L) in dofetilide-induced Torsades de Pointes. J Mol Cell Cardiol. 2004;37:1031–1039. doi: 10.1016/j.yjmcc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Manocha P., Bavikati V., Langberg J. Coronary artery disease potentiates response to dofetilide for rhythm control of atrial fibrillation. Pacing Clin Electrophysiol. 2012;35:170–173. doi: 10.1111/j.1540-8159.2011.03245.x. [DOI] [PubMed] [Google Scholar]


