Abstract
Background
Pancreatic adenosquamous carcinoma (PASC) accounts for only 1-4% of all exocrine pancreatic cancers and carries a particularly poor prognosis. This retrospective study was performed to determine whether inclusion of a platinum agent as part of adjuvant therapy is associated with improved survival in patients with resected PASC.
Methods
Records of all patients who underwent pancreatic resection at Johns Hopkins Hospital from 1986 to 2012 were reviewed to identify those with PASC. Multivariable Cox proportional hazards modeling was used to assess for significant associations between patient characteristics and survival.
Results
In total, 62 patients (1.1%) with resected PASC were identified among 5,627 cases. Median age was 68 [interquartile range (IQR), 57-77] and 44% were female. Multivariate analysis revealed that, among all patients (n=62), the following factors were independently predictive of poor survival: lack of adjuvant therapy [hazard ratio (HR) =3.6; 95% confidence interval (CI), 1.8-7.0; P<0.001], margin-positive resection (HR =3.5; 95% CI, 1.8-6.8; P<0.001), lymph node involvement (HR =3.5; 95% CI, 1.5-8.2; P=0.004), and age (HR =1.0; 95% CI, 1.0-1.1; P=0.035). There were no significant differences between patients who did and did not receive adjuvant therapy following resection (all P>0.05). A second multivariable model included only those patients who received adjuvant therapy (n=39). Lack of inclusion of a platinum agent in the adjuvant regimen (HR =2.4; 95% CI, 1.0-5.8; P=0.040) and larger tumor diameter (HR =1.3; 95% CI, 1.0-1.6; P=0.047) were independent predictors of inferior survival.
Conclusions
Addition of a platinum agent to adjuvant regimens for resected PASC may improve survival among these high-risk patients, though collaborative prospective investigation is needed.
Keywords: Adenosquamous carcinoma, adjuvant therapy, platinum chemotherapy, pancreatic cancer
Introduction
Pancreatic ductal adenocarcinoma is among the most lethal malignancies, responsible for approximately 38,460 deaths in 2013 alone (1). Even for patients with early stage, resectable disease, survival rates remain poor. Due to aggressive tumor biology and likely undetected metastatic disease at the time of resection, only 22% of patients with pancreatic cancer survive beyond 5 years despite modern multimodality treatment approaches (2,3).
Pancreatic adenosquamous carcinoma (PASC) is a rare, yet particularly aggressive subtype of pancreatic adenocarcinoma, accounting for only 1-4% of exocrine pancreatic cancers (4-8). PASC is characterized by the histopathologic presence of both ductal adenocarcinoma and squamous carcinoma elements in a single tumor. Strict classification of PASC is dependent on the presence of ≥30% malignant keratinized squamous cell elements mixed with ductal adenocarcinoma (9), though a lower percentage of squamous component (<30%) in PASC does not appear to be associated with improved clinical prognosis (10). Median overall survival for PASC patients undergoing curative-intent resection has been previously reported to be only 11 months compared to 18 months for resected pure ductal adenocarcinoma at the same institution (11). Additionally, patients with PASC tend to have larger tumors that are more often poorly differentiated with lymph node involvement as compared to patients with pure adenocarcinomas (12).
Optimal management and prognostic factors for improved outcomes are poorly defined give the rarity of this morphological variant (13). Surgical resection appears to improve survival in some studies (7,13,14); however, others report median survivals of less than 6 months following resection (15-17). One large series of PASC patients failed to demonstrate a significant association between the use of adjuvant radiation or chemotherapy and improved survival; however, this study was unable to account for performance status at the time of treatment (12). On the other hand, our group previously demonstrated a survival benefit with the use of chemoradiation therapy following resection in patients with PASC (10). Additional investigations of the efficacy of adjuvant chemoradiation are limited to anecdotal studies (6-8,14,18-20).
The approach to selecting a chemotherapy agent for the treatment of PASC is similar to that for pancreatic adenocarcinoma, employing gemcitabine alone or in combination with capecitabine, erlotinib, 5-fluorouracil (5-FU), or platinum-based antineoplastic agents (21,22). The combination of gemcitabine and platinum-based agents is based on the rationale of a synergistic effect when the two drugs are delivered together. In preclinical studies, cisplatin increased the incorporation of gemcitabine into DNA, inhibiting replication and promoting apoptosis, whereas gemcitabine increased the frequency and inhibited the repair of DNA lesions caused by cisplatin (23-25).
Platinum-based agents have demonstrated improved outcomes in squamous cell cancers in other tumor sites such as the esophagus (26,27), head and neck (28-31), and ovary (32-35). The addition of cisplatin to gemcitabine as first-line therapy for pancreatic cancer, however, failed to demonstrate improved survival in patients with diverse histological subtypes in two Italian randomized trials (36,37). Reports of the efficacy of platinum-based agents for PASC, though promising, have also been limited to case reports (38,39). The goal of this study is to specifically assess whether the addition of a platinum-based antineoplastic agent to the adjuvant treatment regimen is associated with improved survival when compared to standard adjuvant therapy.
Materials and methods
Patient selection
This study was designed as a retrospective chart review approved by the Johns Hopkins Institutional Review Board. The records of all pancreatic cancer patients who underwent surgical resection with curative intent at our institution from 1986 through 2012 were reviewed to identify patients whose pancreatic carcinoma specimens demonstrated any amount of squamous differentiation based on the finalized surgical pathology report. This process identified 69 patients with a pathologic diagnosis of PASC or adenocarcinoma with squamous differentiation (these two entities were considered the same and will, from hereon, be referred to simply as PASC).
These 69 cases comprised only 1.2% of the 5,627 total patients who underwent pancreatectomy for biopsy-proven or presumed pancreatic cancer during the 26-year time period we examined. Study inclusion criteria consisted of the following: age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, documentation of squamous differentiation in the surgical pathology report, localized PASC post resection, and patient follow-up to assess adjuvant therapy. Seven (10%) of the 69 patients with PASC identified by our chart review were excluded from the final analysis—three patients as a result of distant metastasis to the liver during surgical resection and four due to lack of any follow-up after surgical resection. The final study therefore includes 62 patients diagnosed with non-metastatic PASC following curative resection. Of these 62 patients, 48 (77%) had histologic slides available for re-review by a single pathologist to ensure that a squamous component was present; in all 48 cases, pathologic re-review confirmed the presence of squamous differentiation.
Data collection
Demographic, tumor resection specimen, and treatment characteristics were obtained from the electronic medical record and/or paper chart for each patient. Additional data were obtained in regard to potential prognostic factors identified in previously published reports (4-8,10-15,18-20). Patient-specific variables included age, sex, and ECOG performance status. Variables specific to the tumor resection specimen included maximum tumor diameter, histologic grade, tumor (T) stage, lymph node involvement, margin status, lymphovascular invasion, and perineural invasion. Treatment parameters consisted of chemotherapy regimens received (both alone and in combination with radiation, if applicable), type of platinum-based chemotherapy agents received, the number of cycles of platinum-based chemotherapy received (if applicable), and the radiation dose received (if applicable).
Statistical analysis
Demographic, tumor resection specimen, and therapy characteristics were summarized using descriptive statistics. Proportions were compared between groups using Fisher’s exact test, while continuous data (age, tumor diameter, and radiation dose) were compared between groups using the Mann-Whitney U test. Median survival was the primary outcome of interest. Survival for each patient was calculated from the date of histopathologic diagnosis to the date of death. In cases where the subject remained alive at the date of last follow-up, survival was censored at that date. Survival probabilities were estimated using Kaplan-Meier statistics (40). Univariate Cox regression analyses were employed to assess for associations between potential prognostic factors and survival. Factors demonstrating a statistically significant association with survival (P<0.05) or a trend towards association with survival (P<0.10) on univariate analysis, along with factors of accepted clinical importance (age, sex, performance status), were selected as covariates to construct multivariate proportional hazards regression models for survival (41). These models were used to estimate the hazard ratio (HR) for death attributable to each covariate using backward elimination. The a priori level of significance was set at P≤0.05 and P values were reported as two-sided in all cases. Statistical analyses were performed using SPSS software, version 20 (International Business Machines Corporation, Armonk, NY, USA).
Results
Patients
Overall, 62 patients with PASC were included in the analysis. Demographic, baseline disease, and treatment characteristics are summarized in Table 1. The median age of the entire cohort was 68 years, 56% were male, and most patients (79%) had an ECOG performance status of 0 at baseline. Median tumor diameter at resection was 4.0 cm, with most tumors being poorly differentiated (82%), pathologic T3 stage (66%), and demonstrating high-risk features including lymph node involvement (77%), lymphovascular invasion (74%), and perineural invasion (84%). Positive resection margins occurred in 29% of cases and 63% of patients went on to receive adjuvant therapy following resection.
Table 1. Demographic, baseline disease, and treatment characteristics for the entire cohort (n=62) and broken down by whether or not patients received adjuvant therapy following surgical resection.
| Characteristic | All patients (n=62) | Patients who received adjuvant therapy (n=39) | Patients who did not receive adjuvant therapy (n=23) | P value |
|---|---|---|---|---|
| Demographic data | ||||
| Age: median [IQR] | 68 [57-77] | 67 [55-76] | 68 [65-80] | 0.107 |
| Age ≥65 years: No. (%) | 38 [61] | 21 [54] | 17 [74] | 0.177 |
| Gender: No. male (%) | 35 [56] | 23 [59] | 12 [52] | 0.791 |
| ECOG performance status ≥1: No. (%) | 13 [21] | 10 [26] | 3 [13] | 0.338 |
| Tumor resection specimen data | ||||
| Tumor diameter: median centimeters (IQR) | 4.0 (3.0-6.0) | 4.5 (3.5-6.0) | 4.0 (3.0-6.3) | 0.645 |
| Tumor diameter: No. >3 cm [%] | 45 [73] | 30 [77] | 15 [65] | 0.383 |
| Histologic grade | ||||
| No. well differentiated [%] | 0 [0] | 0 [0] | 0 [0] | – |
| No. moderately differentiated [%] | 11 [18] | 6 [15] | 5 [22] | 0.732 |
| No. poorly differentiated [%] | 51 [82] | 33 [85] | 18 [78] | 0.732 |
| Tumor stage | ||||
| No. T2 [%] | 10 [16] | 7 [18] | 3 [13] | 0.731 |
| No. T3 [%] | 41 [66] | 24 [62] | 17 [73] | 0.409 |
| No. T4 [%] | 11 [18] | 8 [21] | 3 [13] | 0.516 |
| Lymph node involvement at resection: No. [%] | 48 [77] | 31 [79] | 17 [74] | 0.755 |
| Positive margin at resection: No. [%] | 18 [29] | 11 [28] | 7 [30] | 0.999 |
| Lymphovascular invasion present: No. [%] | 46 [74] | 28 [72] | 18 [78] | 0.765 |
| Perineural invasion present: No. [%] | 52 [84] | 31 [79] | 21 [54] | 0.298 |
The right-most column shows P values for statistical comparison of each characteristic between patients who did (n=39) and did not (n=23) receive adjuvant therapy in the form of chemoradiation or chemotherapy alone. IQR, interquartile range; No., number; ECOG, Eastern Cooperative Oncology Group; cGy, centiGray.
Survival
Fifty of the 62 patients (81%) had died at the time of the final analysis. Median follow-up was 10.3 months [interquartile range (IQR), 3.9-15.9] from diagnosis among all patients and 10.6 months (IQR, 4.9-20.8) among patients remaining alive at last follow-up. Median survival for the entire cohort (n=62) was 11.0 months [95% confidence interval (CI), 8.7-13.3] (Figure 1A). To better elucidate the factors driving prognosis in PASC, univariate Cox regression analyses were performed to assess for associations between possible prognostic factors and survival (Table 2). Six factors were significantly associated with worse survival on univariate analysis: older age, no receipt of adjuvant therapy, lymph node involvement, positive resection margin, lymphovascular invasion, and perineural invasion (see Table 2 for HRs, 95% CI, and P values associated with each factor). These six factors showing a significant association with survival on univariate analysis along with clinically important factors (sex and ECOG performance status) were used to construct a multivariate proportional hazards model for survival among patients with PASC. Backward elimination yielded the following four factors remaining in the model, listed in order of HR magnitude (Table 2, bottom): lack of adjuvant therapy (HR =3.6; 95% CI, 1.8-7.0; P<0.001), positive margin at resection (HR =3.5; 95% CI, 1.8-6.8; P<0.001), pathologic lymph node involvement at resection (HR =3.5; 95% CI, 1.5-8.2; P=0.004), and age as a continuous variable (HR =1.0; 95% CI, 1.0-1.1; P=0.035). Therefore, survival among our cohort of patients with resected PASC appears to be most strongly influenced by whether or not adjuvant therapy was received (Figure 1B).
Figure 1.
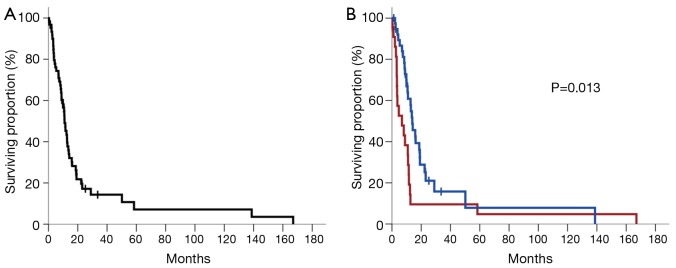
(A) Kaplan-Meier plot of survival for all patients included in the study (n=62); (B) Kaplan-Meier plot of survival stratified by whether patients received adjuvant therapy following tumor resection (blue curve) or not (red curve). Vertical tick marks represent censored patients. The P value is derived from univariate Cox regression analysis.
Table 2. Univariate and multivariate associations between patient characteristics and survival.
| Characteristic | Hazard ratio (95% CI) | P value |
|---|---|---|
| Univariate associations | ||
| Age: ≥65 vs. <65 | 2.1 (1.2-3.9) | 0.014* |
| Age: continuous | 1.0 (1.0-1.1) | 0.009* |
| Gender: female vs. male | 0.9 (0.5-1.6) | 0.771 |
| ECOG: ≥1 vs. 0 | 1.2 (0.6-2.4) | 0.565 |
| Histologic grade: poorly differentiated vs. other | 1.2 (0.6-2.5) | 0.61 |
| Tumor diameter: maximum cm (continuous) | 1.1 (1.0-1.2) | 0.202 |
| Tumor diameter: >3 vs. ≤3 cm | 1.2 (0.6-2.2) | 0.636 |
| Adjuvant therapy received: no vs. yes | 2.1 (1.2-3.8) | 0.013* |
| Lymph node involvement at resection: yes vs. no | 2.3 (1.0-4.9) | 0.038* |
| T stage: T2 vs. ≥T3 | 0.9 (0.4-1.9) | 0.8 |
| Positive margin at resection: yes vs. no | 2.1 (1.1-3.9) | 0.016* |
| Lymphovascular invasion: yes vs. no | 2.2 (1.1-4.5) | 0.027* |
| Perineural invasion: yes vs. no | 3.2 (1.3-8.3) | 0.013* |
| Multivariate associations | ||
| Adjuvant therapy received: no vs. yes | 3.6 (1.8-7.0) | <0.001* |
| Positive margin at resection: yes vs. no | 3.5 (1.8-6.8) | <0.001* |
| Lymph node involvement at resection: yes vs. no | 3.5 (1.5-8.2) | 0.004* |
| Age: continuous | 1.0 (1.0-1.1) | 0.035* |
*, P<0.05. For categorical variables, the second group is the reference group for the Cox proportional hazard analysis. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group.
Adjuvant therapy
Thirty-nine of the 62 patients (63%) received adjuvant therapy following resection. Demographic and treatment characteristics for the adjuvant therapy group are summarized separately in Table 3. In all 39 cases, adjuvant therapy included chemotherapy, which was 5-FU-based only in 15% of patients, gemcitabine-based only in 36%, and included both 5-FU- and gemcitabine-based regimens in 49% due to progression through one regimen type and subsequent salvage therapy with the other. Fourteen patients (36%) received a platinum-based chemotherapy agent for at least two cycles at some point following surgical resection, while 25 patients (64%) did not. Of the 14 patients who received a platinum agent, 10 (71%) received cisplatin and 4 (29%) received oxaliplatin; 12 (86%) received the platinum agent as part of their initial adjuvant chemotherapy regimen following resection whereas 2 (14%) received the platinum agent as part of salvage therapy after developing disease progression on a prior regimen. Specific regimens received included: (I) gemcitabine (600-1,000 mg/m2 weekly)/cisplatin (20-30 mg/m2 weekly) for 9 patients; (II) 5-FU (1,800 mg/m2 continuous infusion biweekly)/irinotecan (120 mg/m2 biweekly)/oxaliplatin (64 mg/m2 biweekly) for 2 patients; (III) capecitabine (1,000 mg PO twice daily)/oxaliplatin (100 mg/m2 biweekly) for one patient; (IV) gemcitabine (1,000 mg/m2 weekly)/oxaliplatin (100 mg/m2 biweekly) for one patient; and (V) cisplatin (80 mg/m2 biweekly)/irinotecan (70 mg/m2 biweekly) for one patient. Median number of cycles received that included a platinum agent was 6 (range, 2-8; IQR, 3-6.5) among these 14 patients. Twenty-five of the 39 patients (64%) also received concurrent chemoradiation as an element of adjuvant therapy, with the median radiation dose received being 5,040 cGy and the concurrent chemotherapy agent being 5-FU or capecitabine in 84% and gemcitabine in 16%.
Table 3. Demographic, baseline disease, and treatment characteristics for the cohort of patients who received adjuvant therapy (n=39) and broken down by whether or not patients received a platinum chemotherapy agent as part of adjuvant therapy following surgical resection.
| Characteristic | All patients who received adjuvant therapy (n=39) | Patients who received a platinum agent for ≥2 cycles (n=14) | Patients who did not receive a platinum agent for ≥2 cycles (n=25) | P value |
|---|---|---|---|---|
| Demographic data | ||||
| Age: median interquartile range [IQR] | 67 [55-76] | 62 [53-69] | 73 [57-77] | 0.128 |
| Age ≥65 years: [%] | 21 [54] | 6 [43] | 15 [60] | 0.337 |
| Male [%] | 23 [59] | 10 [71] | 13 [52] | 0.317 |
| ECOG performance status ≥1 [%] | 10 [26] | 2 [14] | 8 [32] | 0.279 |
| Baseline tumor data | ||||
| Tumor diameter: median centimeters (IQR) | 4.5 (3.5-6.0) | 4.0 (3.1-5.0) | 5.0 (3.5-6.0) | 0.196 |
| Tumor diameter >3 cm [%] | 30 [77] | 10 [71] | 20 [80] | 0.696 |
| Histologic grade | ||||
| Well differentiated [%] | 0 [0] | 0 [0] | 0 [0] | – |
| Moderately differentiated [%] | 6 [15] | 2 [14] | 4 [16] | 0.999 |
| Poorly differentiated [%] | 33 [85] | 12 [86] | 21 [84] | 0.999 |
| Tumor stage | ||||
| T2 [%] | 7 [18] | 3 [21] | 4 [16] | 0.686 |
| T3 [%] | 24 [62] | 8 [57] | 16 [64] | 0.74 |
| T4 [%] | 8 [21] | 3 [21] | 5 [20] | 0.999 |
| Lymph node positive [%] | 31 [79] | 10 [71] | 21 [84] | 0.424 |
| Positive margins [%] | 11 [28] | 2 [14] | 9 [36] | 0.266 |
| Lymphovascular invasion [%] | 28 [72] | 7 [50] | 21 [84] | 0.033* |
| Perineural invasion [%] | 31 [79] | 9 [64] | 22 [88] | 0.109 |
| Treatment data | ||||
| Chemotherapy | 39 [100] | 14 [100] | 25 [100] | – |
| 5-FU based only [%] | 6 [15] | 2 [14] | 4 [16] | 0.999 |
| Gemcitabine based only [%] | 17 [44] | 8 [57] | 10 [40] | 0.314 |
| Included both 5-FU & gemcitabine [%] | 16 [41] | 4 [29] | 12 [48] | |
| Included platinum agent at any point [%] | 14 [36] | 14 [100] | – | – |
| Cycles of platinum received: median (IQR) | – | 6 (3-6.5) | – | – |
| Concurrent chemoradiation [%] | 25 [64] | 10 [71] | 15 [60] | 0.729 |
| 5-FU† [%] | 21 [84] | 9 [64] | 11 [44] | 0.32 |
| Gemcitabine [%] | 4 [16] | 1 [7] | 3 [12] | 0.999 |
| Median radiation dose, cGy [IQR] | 5,040 [5,000-5,040] | 5,040 [5,040-5,040] | 5,040 [5,000-5,040] | 0.603 |
†, includes both infusional 5-fluorouracil and oral capecitabine; *, P<0.05. The right-most column shows P values for statistical comparison of each characteristic between patients who did (n=14) and did not (n=25) receive a platinum chemotherapy agent as part of adjuvant therapy. IQR, interquartile range; No., number; ECOG, Eastern Cooperative Oncology Group; cGy, centiGray; 5-FU, 5-fluorouracil.
Median survival was 13.9 months (95% CI, 10.4-17.3) among patients who received adjuvant therapy (n=39) compared to 6.8 months (95% CI, 0.3-13.3) among patients who did not (n=23; P=0.013) (Figure 1B). There were no significant differences in demographic or baseline disease characteristics between patients who did and did not receive adjuvant therapy following resection (all P>0.05) (Table 1), rendering confounding by any of these variables less likely.
However, the association of adjuvant therapy with improved outcomes could be reflecting a survival bias, as patients who survive sufficiently may be the only ones to undergo adjuvant therapy. To eliminate this potential bias and test our hypothesis that certain antineoplastic therapies may be particularly effective against PASC, survival analysis was repeated for the 39 patients (63%) who received adjuvant therapy. Twenty-nine of the 39 patients (74%) who received adjuvant therapy had died at the time of analysis. Median follow-up was 12.7 months (IQR, 7.9-18.9) from diagnosis among all patients and 10.6 months (IQR, 7.2-18.7) among patients remaining alive at last follow-up. As stated above, the median survival for the entire adjuvant therapy cohort (n=39) was 13.9 months (95% CI, 10.4-17.3) (Figure 2A).
Figure 2.
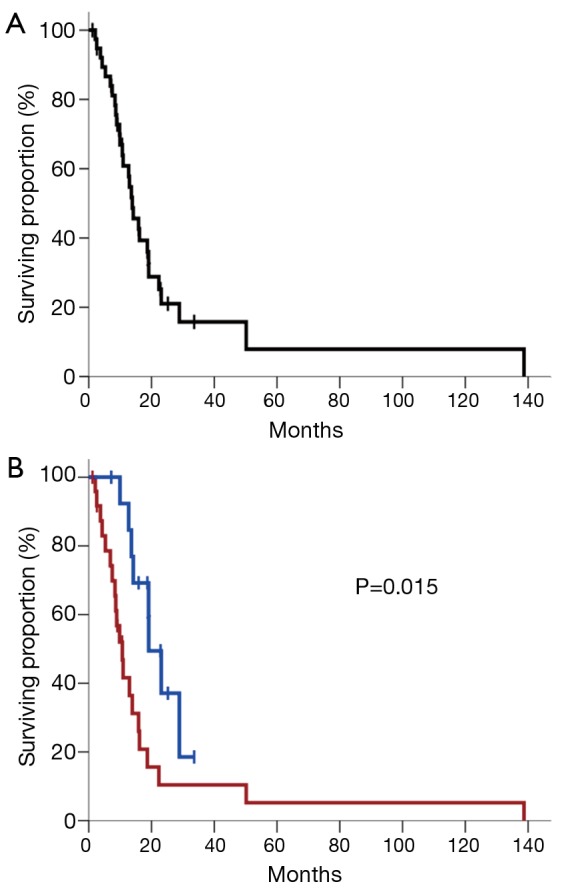
(A) Kaplan-Meier plot of survival for all patients who received adjuvant therapy following tumor resection (n=39); (B) Kaplan-Meier plot of survival stratified by whether patients received ≥2 cycles of a platinum chemotherapy agent as part of their adjuvant therapy regimen (blue curve) or not (red curve). Vertical tick marks represent censored patients. The P value is derived from univariate Cox regression analysis.
To identify specific factors driving prognosis in patients receiving adjuvant therapy for PASC, univariate Cox regression analyses were once again performed to assess for associations between possible prognostic factors and survival (Table 4). Five factors demonstrated a significant association (P<0.05) or a trend towards significant association (P<0.10) with survival on univariate analysis: tumor diameter as a continuous variable, receipt of a platinum agent as part of adjuvant therapy, positive resection margin, lymphovascular invasion, and receipt of chemoradiation as part of adjuvant therapy (see Table 4 for HRs, 95% confidence intervals, and p-values associated with each factor). Whether adjuvant chemotherapy was 5-FU-based only (HR =2.2; 95% CI, 0.7-6.8; P=0.152), gemcitabine-based only (HR =0.6; 95% CI, 0.3-1.5; P=0.298), or included both 5-FU and gemcitabine (HR =1.4; 95% CI, 0.6-3.1; P=0.382) did not significantly influence survival among patients receiving adjuvant chemotherapy (Table 4). The five factors listed above that demonstrated either a significant association or a trend toward significant association with survival along with factors of established clinical importance (age, sex, and ECOG performance status) were incorporated as covariates to create a multivariate proportional hazards model for survival among patients receiving adjuvant therapy for PASC. Backward elimination yielded the following two factors remaining in the model (Table 4, bottom): whether or not a platinum agent was received as part of adjuvant therapy (HR =2.4; 95% CI, 1.0-5.8; P=0.040) and maximum tumor diameter as a continuous variable (HR =1.3; 95% CI, 1.0-1.6; P=0.047).
Table 4. Univariate and multivariate associations between patient characteristics and survival among patients who received adjuvant therapy (n=39).
| Characteristic | Hazard ratio (95% CI) | P value |
|---|---|---|
| Univariate associations | ||
| Age: ≥65 vs. <65 | 1.7 (0.8-3.7) | 0.154 |
| Age: continuous | 1.0 (1.0-1.1) | 0.19 |
| Gender: female vs. male | 0.8 (0.3-1.7) | 0.507 |
| ECOG: ≥1 vs. 0 | 1.2 (0.5-2.7) | 0.751 |
| Histologic grade: poorly differentiated vs. other | 1.1 (0.4-3.3) | 0.827 |
| Tumor diameter: maximum cm (continuous) | 1.3 (1.1-1.7) | 0.015* |
| Tumor diameter: >3 vs. ≤3 cm | 2.2 (0.8-5.7) | 0.123 |
| 5-FU based chemotherapy only: yes vs. no | 2.2 (0.7-6.8) | 0.152 |
| Gemcitabine based chemotherapy only: yes vs. no | 0.6 (0.3-1.5) | 0.298 |
| Both 5-FU and gemcitabine based chemotherapy: yes vs. no | 1.4 (0.6-3.1) | 0.382 |
| Platinum agent received: no vs. yes | 2.8 (1.2-6.6) | 0.015* |
| Lymph node involvement at resection: yes vs. no | 1.9 (0.7-5.5) | 0.243 |
| T stage: T2 vs. ≥T3 | 0.8 (0.3-2.0) | 0.625 |
| Positive margin at resection: yes vs. no | 2.0 (0.9-4.5) | 0.08 |
| Lymphovascular invasion: yes vs. no | 2.5 (1.0-6.3) | 0.051 |
| Perineural invasion: yes vs. no | 2.3 (0.8-6.7) | 0.13 |
| Received chemoradiation: no vs. yes | 2.0 (0.9-4.4) | 0.079 |
| Multivariate associations | ||
| Platinum agent received: no vs. yes | 2.4 (1.0-5.8) | 0.040* |
| Tumor diameter: maximum cm (continuous) | 1.3 (1.0-1.6) | 0.047* |
*, P<0.05. For categorical variables, the second group is the reference group for the Cox proportional hazard analysis. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; 5-FU, 5-fluorouracil.
Consequently, the strongest independent predictor of survival among patients receiving adjuvant therapy for PASC in our cohort was whether or not a platinum agent was received for at least two cycles as part of adjuvant or salvage therapy (Figure 2B). Median survival for the platinum group was 19.1 months (95% CI, 13.8-24.4) compared to 10.7 months (95% CI, 7.9-13.5) for the non-platinum group (P=0.015, log-rank test). Notably, there were no significant differences in demographic, baseline disease, or treatment characteristics between the group of 14 patients who received a platinum agent and the group of 25 patients who did not receive a platinum agent, except that the non-platinum group did have a higher proportion of patients with lymphovascular invasion present in their tumor resection specimens than the platinum group (84% vs. 50%, respectively; P=0.033) (Table 3). Given that the presence of lymphovascular invasion was, however, accounted for by inclusion as a covariate in the multivariate model, it is less likely that this difference between the two groups confounds our results. Survival was not significantly different between the 10 patients who received cisplatin and the four patients who received oxaliplatin (median survival of 19.1 vs. 23.1 months, respectively, P=0.886).
Discussion
PASC is a rare variant of pancreatic adenocarcinoma that is associated with particularly poor survival. Furthermore, few studies have identified prognostic factors to guide the treatment and counseling of patients with PASC. Two of the largest and most recent studies, however, demonstrated an improved median survival among patients who underwent resection compared to those who were unresectable (~12 vs. 5 months, respectively) (12,13). Despite smaller, conflicting reports suggesting that resection does not prolong survival, it is general practice at most major institutions to attempt resection when feasible. Interestingly, the proportion of squamous cell differentiation (<30% vs. ≥30%) also does not appear to be predictive of overall survival (10). Clinicopathologic factors such as tumor size, histologic grade, and resection margin status, which are historically found to be prognostic for patients with resected pure pancreatic adenocarcinoma, had not been previously associated with outcomes in patients with PASC (11,13) perhaps due to the overall aggressive nature of this morphological subtype or the small sample sizes in the previous investigations. Our study is the first to report an association between margin status and tumor size with improved survival among patients receiving adjuvant therapy for PASC.
The role of adjuvant therapy in the management of PASC is also debated. Our group previously reported that adjuvant chemoradiation was associated with improved survival (13.6 months) compared to surgery alone (8.6 months) (10). We reported that adjuvant chemoradiation was associated with improved survival in select subgroups, specifically patients with tumors ≥3 cm in diameter, poor differentiation, lymphovascular or perineural invasion, and squamous component of ≥30%. Adjuvant therapy consisting of either chemotherapy or radiation, however, did not predict improved survival in patients in a larger study (12), likely due to the inability to control for performance status as it was not documented in the dataset used by the authors of the study. Thus, the role of adjuvant therapy has not yet been well defined. Our study makes a convincing case for the value of adjuvant therapy in treating PASC, as the receipt of adjuvant therapy was the strongest independent predictor for improved survival among our cohort of patients.
PASC has been reported to represent approximately 1-4% of all pancreatic neoplasms (4-8), with some estimates as low as 0.4% (12). The 62 patients examined in this study represented only 1.1% of all patients undergoing pancreatectomy for pancreatic cancer at our institution from 1986 to 2012. Unlike patients with pancreatic adenocarcinoma, stage-specific treatment protocols do not exist for PASC given the rarity of the disease and, thus, the difficulty in practicing evidence-based medicine in this histologic subtype. Current evidence for optimal therapy is extremely limited and largely anecdotal; therefore, despite its retrospective design, this study represents a useful body of evidence for guiding adjuvant chemotherapy for PASC.
In this study, the addition of cisplatin or oxaliplatin to gemcitabine- or 5-FU-based adjuvant chemotherapy was associated with significantly longer median survival compared to patients who did not receive a platinum agent. This association was confirmed in a multivariate proportional hazards model that adjusted for age, sex, and performance status as well as other factors showing univariate associations with survival (tumor diameter, positive resection margin, lymphovascular invasion, and receipt of chemoradiation). These findings highlight the need for prospective investigation of platinum-containing adjuvant therapy regimens, and perhaps neoadjuvant regimens if adenosquamous histology is identified on a diagnostic fine-needle aspirate. Given the rarity of this histopathologic entity, multi-institutional collaboration will be necessary to make such investigation possible.
Though the optimal adjuvant regimen for patients with PASC cannot be determined from our results, the majority of patients (10 of 14) who received a platinum agent in our study did so in combination with gemcitabine, suggesting that this dual regimen may hold promise. The rationale for combining gemcitabine and a platinum-based agent for the treatment of PASC includes both clinical and basic laboratory data. Though performed among patients with pancreatic ductal adenocarcinoma, a phase III trial of gemcitabine vs. 5-FU maintenance chemotherapy following 5-FU-based chemoradiation showed a trend towards improved survival with gemcitabine (42). An earlier phase III trial in patients with advanced pancreatic ductal adenocarcinoma showed first-line gemcitabine chemotherapy yielded superior survival outcomes and clinical benefit (improved performance status and pain) compared to first-line 5-FU (43).
This study has several limitations. The retrospective design leads to potential inherent biases. As there was no standardization of chemotherapy doses and duration as well as a heterogeneous approach to salvage therapies, we are limited in the ability to recommend a specific chemotherapy regimen for management of PASC. Given the rarity of PASC, this single-institution study is small with only 62 patients. As a result, the study may be underpowered to definitively identify all significant prognostic factors among those examined. For instance, receipt of adjuvant chemoradiation has been previously associated with significantly improved survival among patients with PASC (10,12). In our study, chemoradiation demonstrated a trend towards association with survival on univariate analysis, but not in the final multivariate proportional hazards model, likely due to the small number of patients and resulting lack of power to determine this association. Although this is the largest report on PASC, collaborative prospective investigation among large-volume centers is needed.
Conclusions
Independent predictors for survival among patients with PASC include receipt of adjuvant therapy, margin and lymph node status at resection, and patient age. Among patients who receive adjuvant therapy, the addition of a platinum agent to 5-FU- or gemcitabine-based chemotherapy regimens appears to independently predict for improved survival. Given the lack of standard chemotherapy regimens for PASC, these findings suggest that an adjuvant combination chemotherapy regimen that includes gemcitabine and a platinum agent with or without radiation therapy could significantly improve outcomes for patients with this aggressive variant of pancreatic cancer and merits prospective investigation.
Acknowledgements
Presented at the American Society of Clinical Oncology (ASCO) Annual Meeting, June 1-4, 2013, Chicago, IL.
Funding: Claudio X. Gonzalez Family Foundation, Flannery Family Foundation, Alexander Family Foundation, Keeling Family Foundation, DeSanti Family Foundation, McKnight Family.
Disclosure: The authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A.Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 2.Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100, 313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg 1999;189:1-7. [DOI] [PubMed] [Google Scholar]
- 3.Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2012;10:703-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cihak RW, Kawashima T, Steer A. Adenoacanthoma (adenosquamous carcinoma) of the pancreas. Cancer 1972;29:1133-40. [DOI] [PubMed] [Google Scholar]
- 5.Cubilla AL, Fitzgerald PJ. Morphological patterns of primary nonendocrine human pancreas carcinoma. Cancer Res 1975;35:2234-48. [PubMed] [Google Scholar]
- 6.Hsu JT, Yeh CN, Chen YR, et al. Adenosquamous carcinoma of the pancreas. Digestion 2005;72:104-8. [DOI] [PubMed] [Google Scholar]
- 7.Kardon DE, Thompson LD, Przygodzki RM, et al. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol 2001;14:443-51. [DOI] [PubMed] [Google Scholar]
- 8.Madura JA, Jarman BT, Doherty MG, et al. Adenosquamous carcinoma of the pancreas. Arch Surg 1999;134:599-603. [DOI] [PubMed] [Google Scholar]
- 9.Hruban RH, Pitman MB, Klimstra DS. Tumors of the pancreas. AFIP Atlas of Tumor Pathology 2007:177-81. [Google Scholar]
- 10.Voong KR, Davison J, Pawlik TM, et al. Resected pancreatic adenosquamous carcinoma: clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum Pathol 2010;41:113-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz MH, Taylor TH, Al-Refaie WB, et al. Adenosquamous versus adenocarcinoma of the pancreas: a population-based outcomes analysis. J Gastrointest Surg 2011;15:165-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd CA, Benarroch-Gampel J, Sheffield KM, et al. 415 patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res 2012;174:12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smoot RL, Zhang L, Sebo TJ, et al. Adenosquamous carcinoma of the pancreas: a single-institution experience comparing resection and palliative care. J Am Coll Surg 2008;207:368-70. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi K, Enjoji M.Adenosquamous carcinoma of the pancreas: a clinicopathologic study. J Surg Oncol 1991;47:109-16. [DOI] [PubMed] [Google Scholar]
- 16.Brody JR, Costantino CL, Potoczek M, et al. Adenosquamous carcinoma of the pancreas harbors KRAS2, DPC4 and TP53 molecular alterations similar to pancreatic ductal adenocarcinoma. Mod Pathol 2009;22:651-9. [DOI] [PubMed] [Google Scholar]
- 17.Motojima K, Tomioka T, Kohara N, et al. Immunohistochemical characteristics of adenosquamous carcinoma of the pancreas. J Surg Oncol 1992;49:58-62. [DOI] [PubMed] [Google Scholar]
- 18.Hsu JT, Chen HM, Wu RC, et al. Clinicopathologic features and outcomes following surgery for pancreatic adenosquamous carcinoma. World J Surg Oncol 2008;6:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaue H, Tanimura H, Onishi H, et al. Adenosquamous carcinoma of the pancreas: successful treatment with extended radical surgery, intraoperative radiation therapy, and locoregional chemotherapy. Int J Pancreatol 2001;29:53-8. [DOI] [PubMed] [Google Scholar]
- 20.Matsubayashi H, Matsunaga K, Uesaka K, et al. Pancreatic adenosquamous carcinoma with 7-year survival: a case report and literature review. J Dig Dis 2013;14:207-10. [DOI] [PubMed] [Google Scholar]
- 21.Trikudanathan G, Dasanu CA. Adenosquamous carcinoma of the pancreas: a distinct clinicopathologic entity. South Med J 2010;103:903-10. [DOI] [PubMed] [Google Scholar]
- 22.Wolfgang CL, Herman JM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin 2013;63:318-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergman AM, Ruiz van Haperen VW, Veerman G, et al. Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res 1996;2:521-30. [PubMed] [Google Scholar]
- 24.van Moorsel CJ, Pinedo HM, Veerman G, et al. Mechanisms of synergism between cisplatin and gemcitabine in ovarian and non-small-cell lung cancer cell lines. Br J Cancer 1999;80:981-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang LY, Li L, Jiang H, et al. Expression of ERCC1 antisense RNA abrogates gemicitabine-mediated cytotoxic synergism with cisplatin in human colon tumor cells defective in mismatch repair but proficient in nucleotide excision repair. Clin Cancer Res 2000;6:773-81. [PubMed] [Google Scholar]
- 26.Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [DOI] [PubMed] [Google Scholar]
- 27.Enzinger PC, Ilson DH, Kelsen DP. Chemotherapy in esophageal cancer. Semin Oncol 1999;26:12-20. [PubMed] [Google Scholar]
- 28.Bae WK, Hwang JE, Shim HJ, et al. Multicenter phase II study of weekly docetaxel, cisplatin, and S-1 (TPS) induction chemotherapy for locally advanced squamous cell cancer of the head and neck. BMC Cancer 2013;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz DL, Montgomery RB, Yueh B, et al. Phase I and initial phase II results from a trial investigating weekly docetaxel and carboplatin given neoadjuvantly and then concurrently with concomitant boost radiotherapy for locally advanced squamous cell carcinoma of the head and neck. Cancer 2005;103:2534-43. [DOI] [PubMed] [Google Scholar]
- 30.Amrein PC, Weitzman SA. Treatment of squamous-cell carcinoma of the head and neck with cisplatin and 5-fluorouracil. J Clin Oncol 1985;3:1632-9. [DOI] [PubMed] [Google Scholar]
- 31.Rabbani A, Hinerman RW, Schmalfuss IM, et al. Radiotherapy and concomitant intraarterial cisplatin (RADPLAT) for advanced squamous cell carcinomas of the head and neck. Am J Clin Oncol 2007;30:283-6. [DOI] [PubMed] [Google Scholar]
- 32.Lambert HE, Berry RJ. High dose cisplatin compared with high dose cyclophosphamide in the management of advanced epithelial ovarian cancer (FIGO stages III and IV): report from the North Thames Cooperative Group. Br Med J (Clin Res Ed) 1985;290:889-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neijt JP, ten Bokkel Huinink WW, van der Burg ME, et al. Randomised trial comparing two combination chemotherapy regimens (Hexa-CAF vs CHAP-5) in advanced ovarian carcinoma. Lancet 1984;2:594-600. [DOI] [PubMed] [Google Scholar]
- 34.Williams CJ, Mead GM, Macbeth FR, et al. Cisplatin combination chemotherapy versus chlorambucil in advanced ovarian carcinoma: mature results of a randomized trial. J Clin Oncol 1985;3:1455-62. [DOI] [PubMed] [Google Scholar]
- 35.Omura G, Blessing JA, Ehrlich CE, et al. A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma. A Gynecologic Oncology Group Study. Cancer 1986;57:1725-30. [DOI] [PubMed] [Google Scholar]
- 36.Colucci G, Giuliani F, Gebbia V, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer 2002;94:902-10. [PubMed] [Google Scholar]
- 37.Colucci G, Labianca R, Di Costanzo F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol 2010;28:1645-51. [DOI] [PubMed] [Google Scholar]
- 38.Aurilio G, Macarulla T, Ramos JF, et al. Successful treatment with GEMOX in patient with metastatic pancreatic adenosquamous carcinoma. Tumori 2011;97:239-42. [DOI] [PubMed] [Google Scholar]
- 39.Shibagaki K, Fujita K, Nakayama S, et al. Complete response of a pancreatic adenosquamous carcinoma to chemoradiotherapy. Int J Clin Oncol 2008;13:74-7. [DOI] [PubMed] [Google Scholar]
- 40.Hu XJ, Lagakos SW. Nonparametric estimation of the mean function of a stochastic process with missing observations. Lifetime Data Anal 2007;13:51-73. [DOI] [PubMed] [Google Scholar]
- 41.Cox DR. Regression models and life-tables. J R Stat Soc Ser 1972;34:187-220. [Google Scholar]
- 42.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019-26. [DOI] [PubMed] [Google Scholar]
- 43.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [DOI] [PubMed] [Google Scholar]


