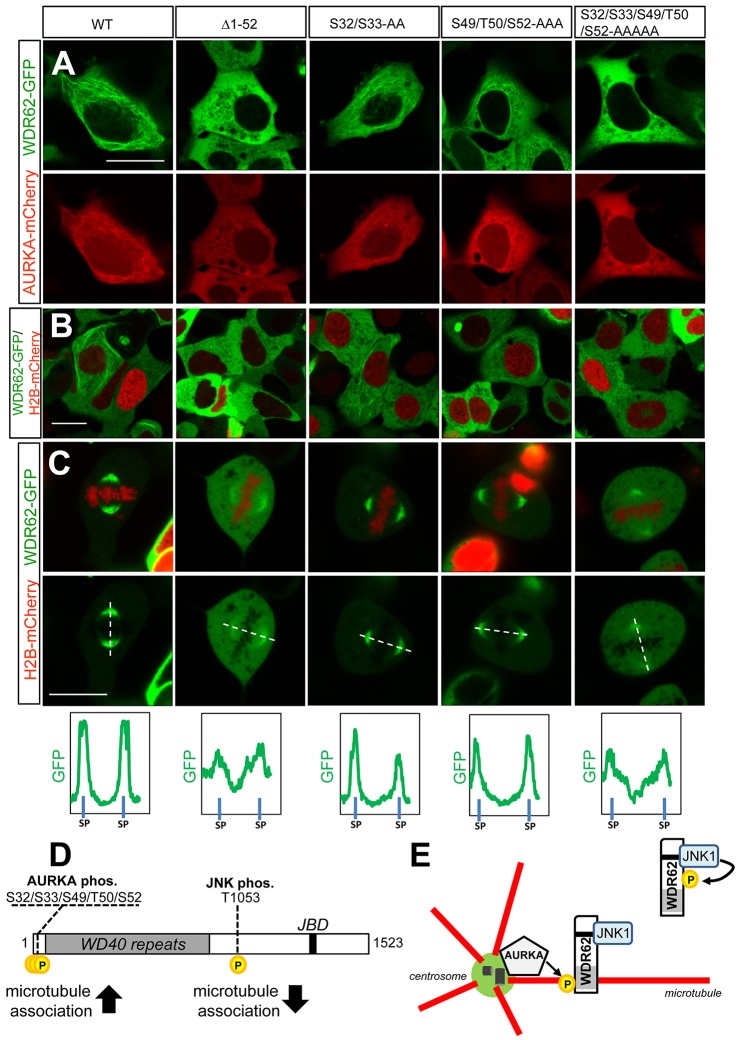
Fig. 7.
AURKA-mediated phosphorylation of residues within the WDR62 N-terminal region regulates microtubule association. (A) GFP-tagged N-terminally truncated (Δ1–52) WDR62, full-length wild-type (WT) WDR62, WDR62 S32/S33-AA, WDR62 S49/T50/S52-AAA or WDR62 S32/S33/S49/T50/S52-AAAAA mutants were coexpressed with mCherry–AURKA and localization was evaluated. (B) GFP-tagged N-terminally truncated or alanine-substituted WDR62 mutants were coexpressed with H2B–mCherry and taxol treated (10 µM, 30 min), and protein localization was evaluated. (C) GFP-tagged N-terminally truncated or alanine-substituted WDR62 mutants were coexpressed with H2B–mCherry and the spindle pole association of WDR62 proteins was determined in mitotic cells. Relative GFP signal intensities were quantified along line scans bisecting spindle poles (SP). Scale bars: 20 µm. (D) Schematic of WDR62 depicting the position of AURKA and JNK phosphorylation sites and their respective effects on protein association with microtubules. (E) Proposed model of WDR62 spatiotemporal regulation by JNK and AURKA. Centrosome-associated AURKA phosphorylates WDR62 to trigger association with astral microtubules following mitotic entry. This is required for the accumulation of WDR62–JNK signaling complexes at the spindle pole. JNK-mediated phosphorylation of WDR62 promotes dissociation from microtubules for cytoplasmic localization.