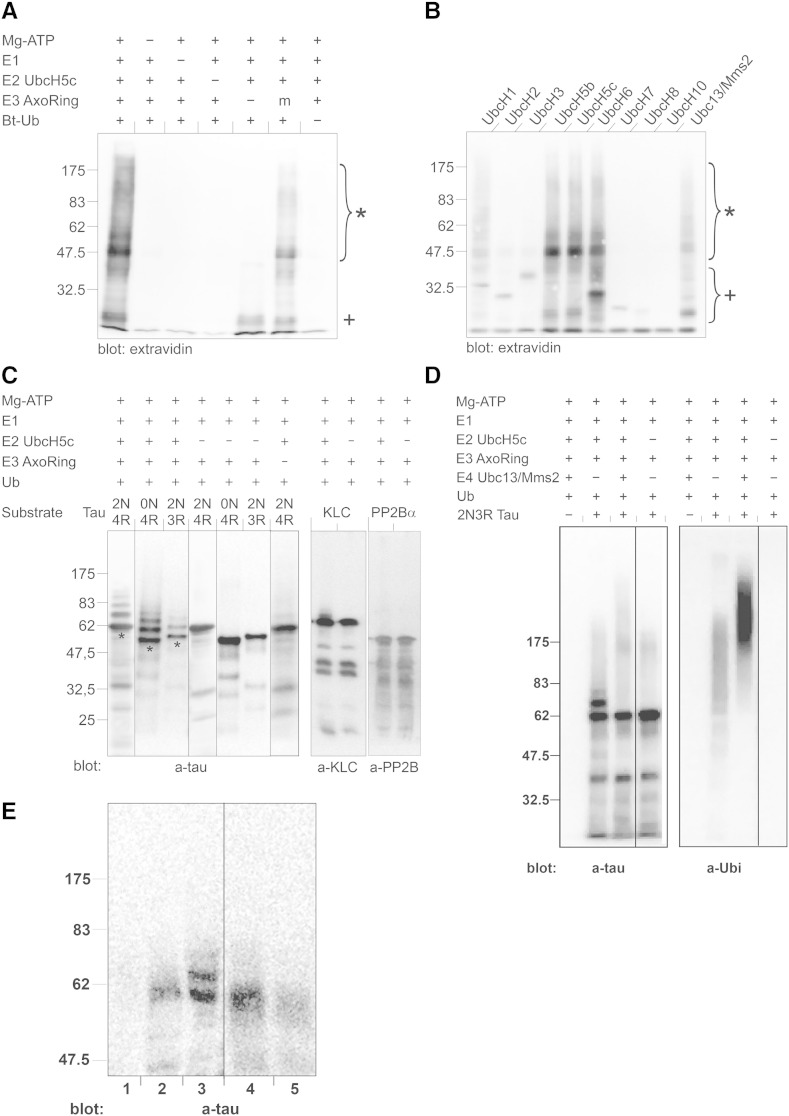
Fig. 3.
Reconstitution of axotrophin ubiquitin ligase activity after recombinant expression of the axotrophin C-terminus comprising the RING-variant domain in E. coli. (A) Testing of E3 auto-ubiquitinating activity of axotrophin RING-variant domain (AxoRing) by Western blotting. We used the UbcH5c E2 enzyme identified in the yeast two-hybrid assay to show that E3 auto-ubiquitinating activity of the RING-variant domain is dependent on all components of the ubiquitination cascade such as the presence of ATP, E1, E2 and ubiquitin. E3 auto-ubiquitinating activity of the axotrophin RING-variant domain is strongly diminished if the first two cysteines of the domain are substituted by serine residues (m, 552CRIC/552SRIS). The use of biotinylated ubiquitin (Bt-Ub) allowed the detection of ubiquitinated proteins by extravidin-HRP. (B) Screening for additional E2 enzymes capable to reconstitute axotrophin E3 ligase activity. In agreement with the yeast two-hybrid analysis the UbcH5 family was identified as the E2 enzymes conferring the highest enzymatic activity to axotrophin. In addition axotrophin E3 ligase activity was also dependent on UbcH6 and to a lesser extent on Ubc13, already identified by yeast two-hybrid analysis. UbcH1 had still has residual activity. *: auto-ubiquitinated axotrophin, +: ubiquitinated E2 enzymes. (C) Axotrophin RING-variant domain ubiquitinates tau in vitro. Axotrophin RING-variant domain (AxoRing) was incubated with Mg-ATP, E1, E2 UbcH5c, ubiquitin and biotinylated human tau isoforms 2N4R, 0N4R, 2N3R or two other axotrophin-interacting proteins KLC1 or PP2Ba. After separating proteins via SDS-PAGE, immunoblotting using an anti-tau, anti-KLC1 or anti-PP2B was done. Lane 1 shows that ubiquitination of the longest human tau isoform 2N4R (62 kDa) produced four additional immunoreactive bands each shifted 7–8 kDa apart in addition to the unmodified protein (asterisk). In the control reactions lacking E2 enzyme (lanes 4–6) and E3 enzyme (lane 7) tau protein isoforms run unmodified as a single band. Lane 2 shows ubiquitination of 0N4R tau isoform, inducing four additional immunoreactive bands. The unmodified 0N4R isoform in lane 5 runs at 55 kDa. Ubiquitination of the human 2N3R tau isoform (that includes the N-terminal inserts but only three instead of four C-terminal microtubule binding repeats) in lane 3 produces only two additional tau bands. The unmodified 2N3R isoform in lane 6 runs at 58 kDa. Complete ubiquitination cascade with E2 UbcH5c leads to additional tau bands above the unmodified tau band, which are shifted each by about 7–8 kDa and represent ubiquitinated tau (lanes 1–3). Employing KLC1 or PP2Ba in the ubiquitination assay as substrates does not result in ubiquitin incorporation in these axotrophin-interacting proteins (compare lanes 8 and 9 and lanes 10 and 11). Ub: ubiquitin, molecular weight is indicated left in kDa. (D) Ubiquitin chain elongation of mono-ubiquitinated tau protein by E4-ligase Ubc13/Mms2. Depletion of shifted 2N3R tau bands in the presence of Ubc13/Mms2 (compare lanes 2 and 3) and increase in high-molecular weight tau smear, which is more visible with anti-ubiquitin antibody (compare lanes 6 and 7), which preferentially labels poly-ubiquitinated tau protein. There is some polyubiquitinated tau protein in the absence of Ubc13/Mms2 (lane 6). (E) Microtubule-binding assay of ubiquitinated 2N4R-tau. 2N4R-tau was ubiquitinated in the presence of Mg-ATP, E1, E2: UbcH5c, E3: axotrophin RING-variant domain and ubiquitin (lanes 2 and 3) or left unmodified by omission of UbcH5 in the reaction mix (lanes 4 and 5). Microtubule assembly in the presence of ubiquitinated 2N4R-tau or non-ubiquitinated 2N4R tau was performed and microtubules were pelleted. Lane 1 shows the absence of tau immunoreactivity in the microtubule pellet, when no exogenous tau is added. Tau species in supernatant (lanes 3 and 5) and microtubule pellet (lanes 2 and 4) were detected by anti-tau immunoblotting. Ubiquitinated tau, visible by shifted bands was detected mostly in the supernatant and not in the microtubule pellet.