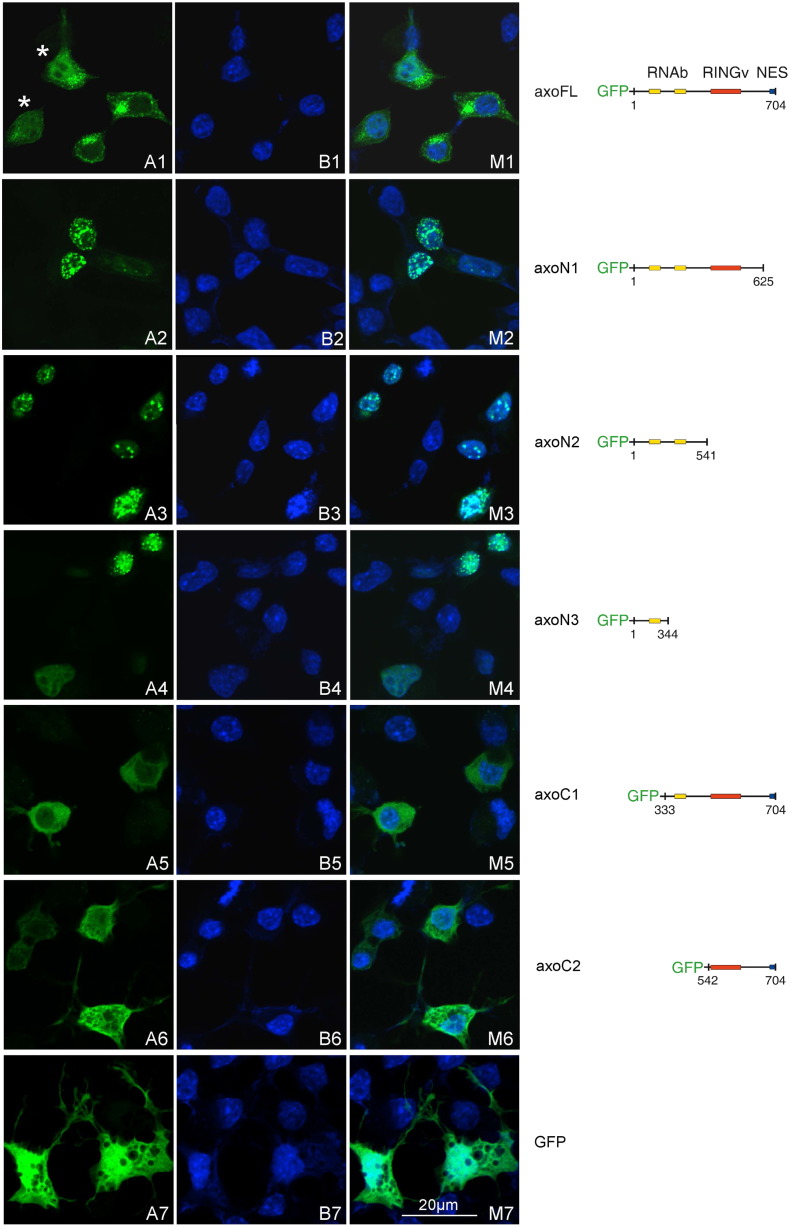
Fig. 6.
Subcellular localization of EGFP–axotrophin fusion proteins. N2A cells were transfected with constructs encoding different axotrophin domains and full length axotrophin as EGFP-fusion proteins. Confocal images of EGFP fluorescence (A1–A7), nuclear DAPI fluorescence (B1–B7) and merge EGFP- and DAPI-fluorescence (M1–M7) were obtained by laser scanning microscopy. Axotrophin expressed as full length protein has a cytoplasmic and nuclear localization (A1, B1). In the cytoplasm axotrophin resides in granular structures in close proximity to the Golgi apparatus. In addition picture A1 shows two cells (marked with asterisk) with more prominent nuclear localization. Upon deletion of aa 626–704 axotrophin exhibits a predominant nuclear localization in a nuclear speckle-like appearance (A2, B2). This nuclear speckle-like localization does change when further amino acids were removed from the C-terminal end such as deleting the RING-variant domain leading to a more heterochromatin associated localization (A3, B3), visible by the large overlap with DAPI stained nuclear foci. After deletion the complete C-terminal half yielding a 344aa construct comprising only the first of two putative DNA/RNA binding domains the nuclear axotrophin localization is confined to smaller nuclear subcompartments (A4, B4). In contrast deletion of aa 1–332, removing the first of two putative DNA/RNA binding domains (A5, B5) results in a predominant cytoplasmic localization without the granular appearance observed with full-length axotrophin (A1, B1). Further deletion of the second putative DNA/RNA binding domain resulting in a construct comprising the RING-variant domain and the C-terminus leads to a cytoplasmic localization of the EGFP-fusion protein with a reticular occurrence (A6, B6). For comparison the dual appearance of solely EGFP in the nuclear and cytoplasmic compartments is shown (A7, B7).