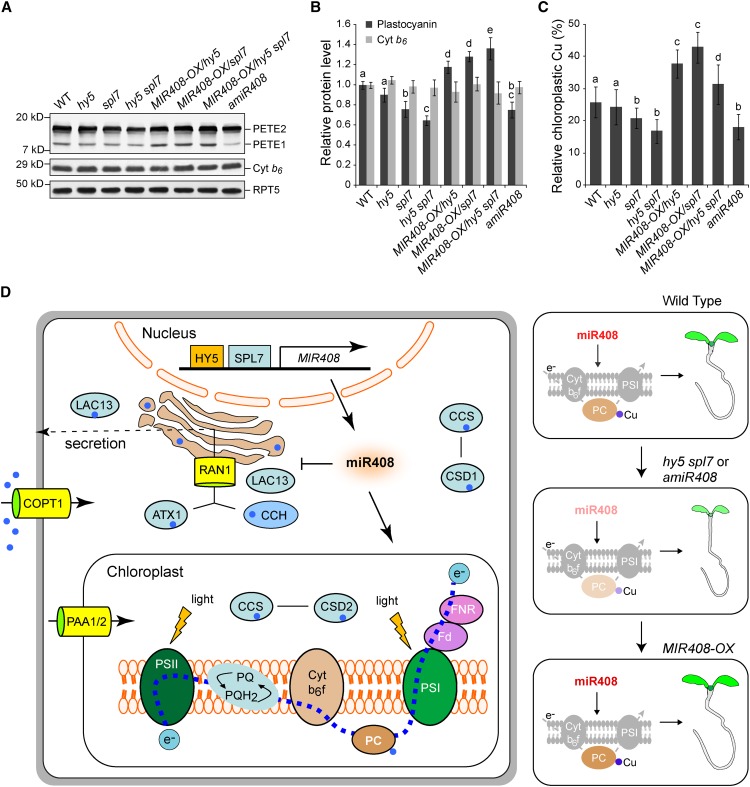
Figure 8.
Cellular Function of miR408.
(A) Detection of PC isoforms in wild-type Arabidopsis seedlings and various genotypes with altered MIR408 expression. Total protein prepared from seedlings grown in the DC/HL condition was fractionated and probed with a commercial PC antibody. Two PC isoforms, PETE1 and PETE2, were detected, with PETE2 being the more abundant isoform. Chloroplast cytochrome b6 (Cyt b6) and RPT5 were used as controls. Blots shown are from one representative of three independent experiments.
(B) Quantification of total PC protein levels in seedlings of various genotypes. The intensity of the bands corresponding to PETE1 and PETE2 was acquired using ImageJ to determine the total PC level, which was then normalized against RTP5 and set to 1 for the wild type. The cytochrome b6 level was also quantified as a control. Data are means ± sd (n = 3).
(C) Relative copper contents in the chloroplast of various genotypes as indicated. Copper content in isolated chloroplasts and whole seedlings of the same genotype was determined separately. Values shown are percentages of chloroplastic copper contents over those of the whole seedlings. Data are means ± sd (n = 4).
Genotypes in (B) and (C) labeled with the same letters have no statistical difference, while different letters denote groups with significant differences (ANOVA, P < 0.01).
(D) A model for SPL7-HY5-regulated MIR408 activation in copper homeostasis and plant development. Depicted on the left are simplified copper transport and utilization pathways that include the main copper transporter COPT1 and the metallochaperones CCS, CCH, and ATX1. These chaperones deliver copper to different internal transporters such as Golgi-localized RNA1 and the chloroplast-specific importers PAA1 and PAA2. Together, the chaperones and transporters mediate copper delivery to specific protein targets such as CSD1 in the cytosol, CSD2 in the chloroplast, PC in the photosynthetic electron transport chain, and LAC13 in the secretory pathway. Elevated miR408, which is promoted by HY5 and SPL7, represses several genes in the copper secretory pathway. Genetically, as shown in the three panels on the right, silencing miR408 expression (amiR408) suppresses PC, reduces chloroplast copper, and compromises seedling development, phenotypes reminiscent of the hy5 spl7 double mutant. Conversely, constitutive action of miR408 in the mutant backgrounds complements such phenotypes. Thus, the cellular function of miR408 is to promote the copper allocation to as well as the abundance of PC, thereby constituting one specific regulatory route downstream of SPL7 and HY5, although its impact on other components of copper homeostasis remains to be determined.