Figure 3.
3D Structures of the Bilin Binding Region in Phys.
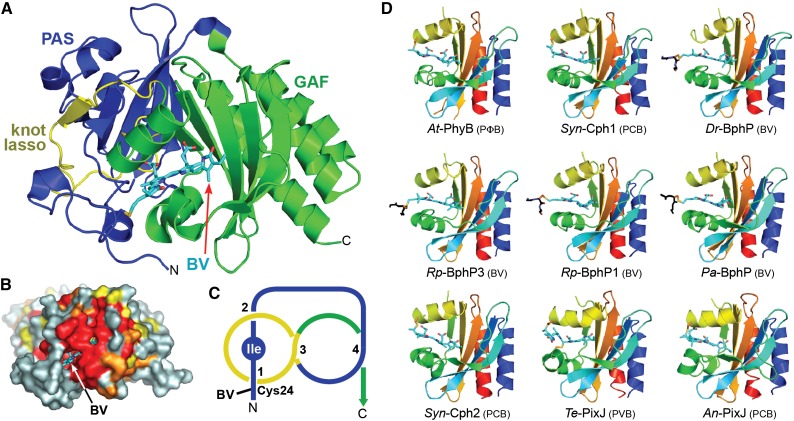
(A) Ribbon diagram of the PAS-GAF region of Dr-BphP (see Protein Databank [PDB] codes 1ZTU, 2O9C, and 4Q0H). Shown are the PAS (blue) and GAF (green) domains and the knot lasso (yellow). BV (arrow) is displayed in cyan with the nitrogens and oxygens colored in blue and red, respectively. N, N terminus; C, C terminus. The sulfur moiety in Cys-24 that forms the thioether linkage with BV is in yellow.
(B) Surface view of the PAS-GAF region of Dr-BphP showing the buried chromophore. Residues with 90, 75, and 60% identity within the Phy superfamily are colored in red, orange, and yellow, respectively. BV (arrow) is displayed in cyan.
(C) Diagram of the figure-of-eight knot connecting the PAS (blue) and GAF domains (yellow) in Dr-BphP. The isoleucine at the nexus of the knot is labeled.
(D) Ribbon diagrams of GAF domains in their dark-adapted states from representative canonical Phys (At-PhyB, PDB code 4OUR; Syn-Cph1, 2VEA; and Dr-BphP, 2O9C), bathy-Phys (Pa-BphP, 3C2W; Rp-BphP1, 4GW9), a Pnr Phy (Rp-BphP3, 2OOL), a PAS-less Phy (Syn-Cph2, 4BWI), and CBCRs (Te-PixJ, 4GLQ; An-PixJ, 3W2Z). Polypeptide chains are displayed in rainbow with the N-terminal ends in blue and the C-terminal ends in red. Bilins are colored in cyan, and the cysteine(s) forming the thioether linkage(s) are in orange with the sulfur atoms in yellow. A portion of the polypeptide for proteobacterial Phys that adjoins the site of thioether linkage is included and shown in black.
([A] and [B] are adapted from Wagner et al. [2005], Figures 1B and 4A.)