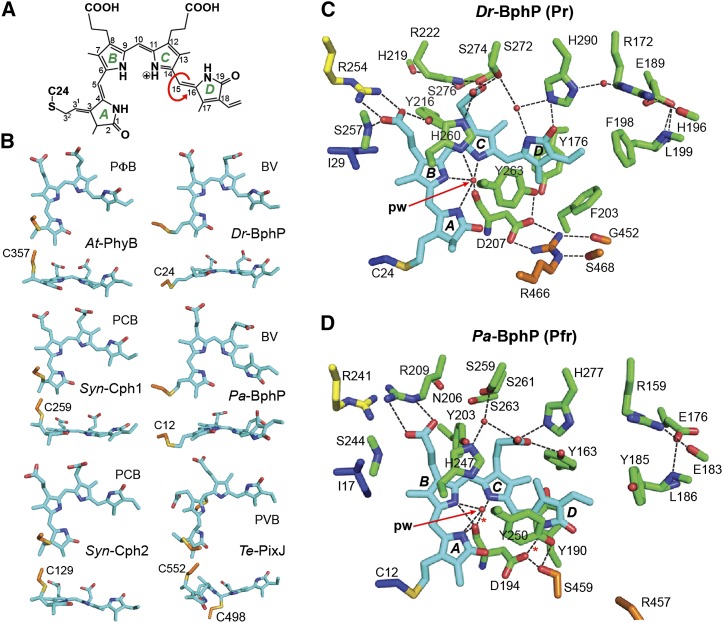
Figure 4.
Structure of the Bilin and Bilin Binding Pocket in Representative Phys.
(A) Chemical diagram of BV bound to Dr-BphP. The pyrrole rings (A-D), carbon positions, and location of the Cys-24 linkage are labeled. Arrow shows the location of the prototypic Z to E isomerization of the bilin during photoconversion.
(B) Conformation in side and top views of the bilin bound to representative Phys in their dark-adapted states. The bilin type is indicated for each Phy. The cysteine(s) that links the bilin via a thioether bond is shown.
(C) and (D) Bilin and surrounding amino acids in the canonical Phy Dr-BphP (C) and the bathy-Phy Pa-BphP (D) in their dark-adapted Pr and Pfr end states, respectively (PDB codes 4Q0J and 3C2W). Amino acids in the PAS domain and upstream region, GAF domain, PHY domain, and the knot lasso are indicated in blue, green, orange, and yellow, respectively. The sulfur moiety in the cysteine forming the thioether linkage with the bilin is in yellow. Fixed waters are in red spheres. pw, pyrrole water. Dashed lines indicate hydrogen bonds.