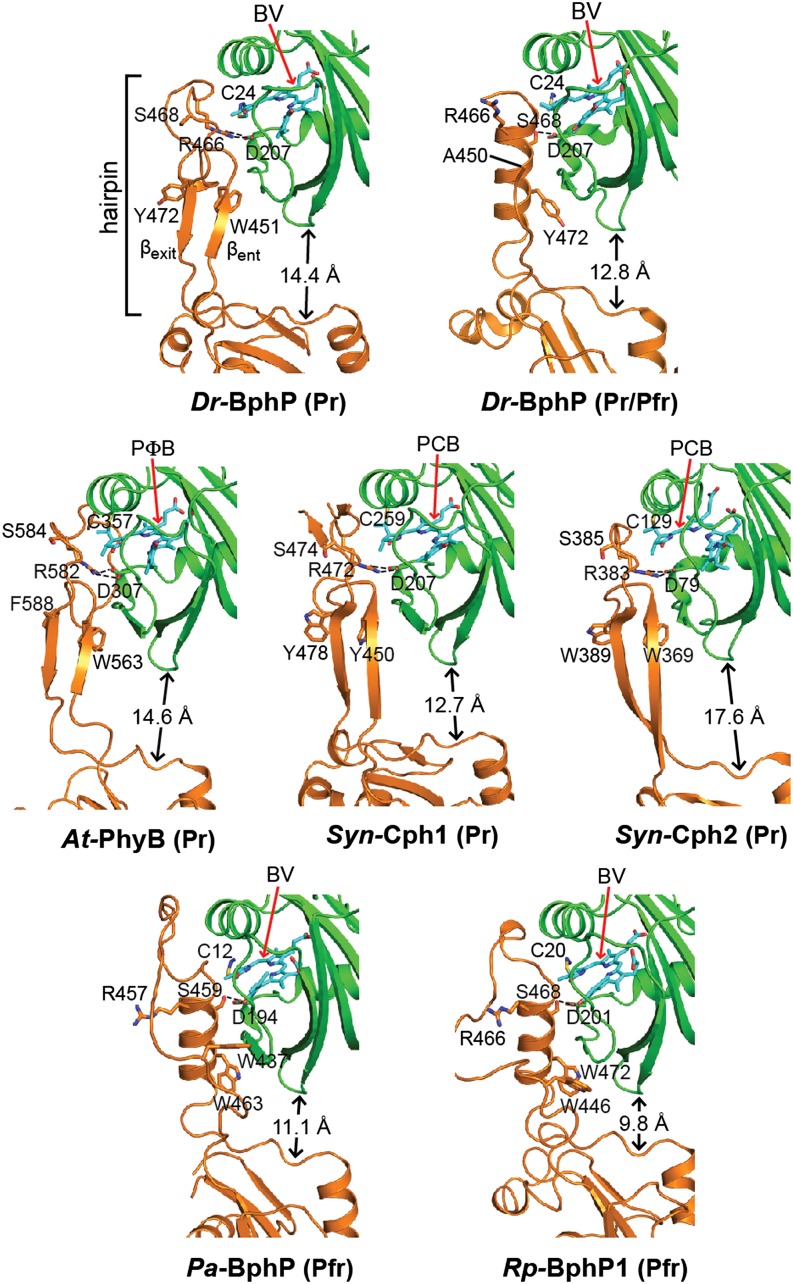
Figure 6.
Conformation of the PHY-Domain Hairpin from Representative Phys and Its Interaction with the GAF Domain.
Ribbon diagrams of crystal structures were drawn from Dr-BphP as Pr (PDB ID code 4Q0J) and a mixed Pr/Pfr state (4O01), Syn-Cph1 as Pr (2VEA), Syn-Cph2 as Pr (4BWI), At-PhyB as Pr (4OUR), Pa-BphP as Pfr (3C2W), and Rp-BphP1 (4GW9) as Pfr. The bilin type (arrow) and the spectral state of each parent model are indicated. The coloring is as in Figure 3. Side chains are shown for relevant amino acids. Dashed lines highlight hydrogen bond contacts between the DIP (Asp-Ile-Pro) motif aspartate in the GAF domain and either the conserved arginine or serine residues in the PRXSF motif from the hairpin stem. The distance separating the GAF and PHY domain globular regions is shown; it was measured from the loop separating the β1 and β2 strands of the GAF domain and the α carbon of a conserved tryptophan (Trp-483 in Dr-BphP) just proximal to the exiting α-helix of the PHY domain. βent and βexit label the entrance (N-terminal) and exit (C-terminal) β-strands in the hairpin. (Adapted from Burgie et al. [2014b], Figure 6.)