Figure 7.
Conformational Changes Associated with Phy Photoconversion.
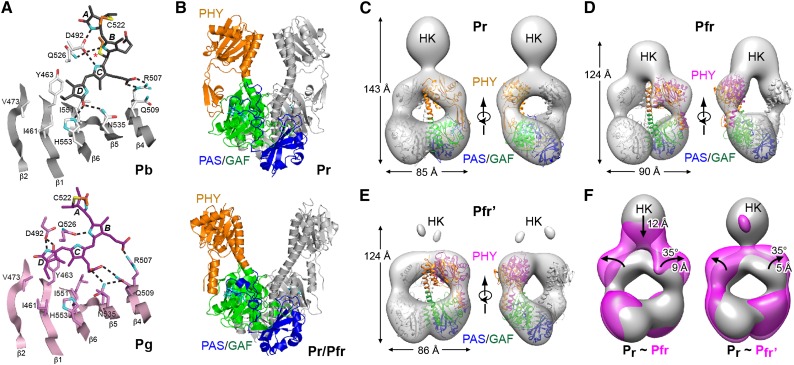
(A) Photoconversion of Te-PixJ from Pb to Pg drives bilin sliding within the GAF pocket. Shown are portions of the GAF domain β-sheet that impinges upon the bilin. Selected residues that contact the bilin directly are labeled. Hydrogen bonds are indicated with dashed lines. Pb and Pg carbons are colored white and pink, respectively, with the exception that cysteine carbons of the thioether linkages are colored orange. Carbons of Pb and Pg state bilins are colored gray and magenta, respectively. Oxygens, red; nitrogens, cyan; sulfurs, yellow. Asterisk identifies the light-labile thioether linkage.
(B) Paired Pr (4Q0J) and Pr/Pfr mixed structures (4O01) of the PSM from Dr-BphP showing the PAS (blue), GAF (green), and PHY (orange) domains.
(C) to (E) Negative-staining SPEM images of full-length Dr-BphP in its dark-adapted Pr and photoactivated Pfr end states. The crystal structures of the corresponding Pr (PDB ID code 4Q0J) and red-light-treated states (4O01) were superposed on the Pr (C) and Pfr/Pfr’ models ([D] and [E]). Each micrograph is shown in two orientations, and height and width of the respective species are indicated. Domain coloring of the crystal structures is the same as in (B). The positions of the PHY domains in (D) and (E) were adjusted to better fit the SPEM density (magenta).
(F) Superimposed SPEM density of the Pr versus the Pfr (left) and Pfr’ conformers (right). The position of the histidine kinase domain is indicated (HK). Arrows highlight the direction of displacement of the PHY or HK domains from the Pr state to the Pfr or Pfr’ states.
([A] is adapted from Cornilescu et al. [2014], Figure 4; [B] from Takala et al. [2014], Figure 2A, and Burgie et al. [2014b], Figure 5A; and [C] to [F] from Burgie et al. [2014b], Figures 7C to 7F.)