Figure 9.
Toggle Model for Photoconversion of Canonical Phys That Translates Light into a Conformational Signal.
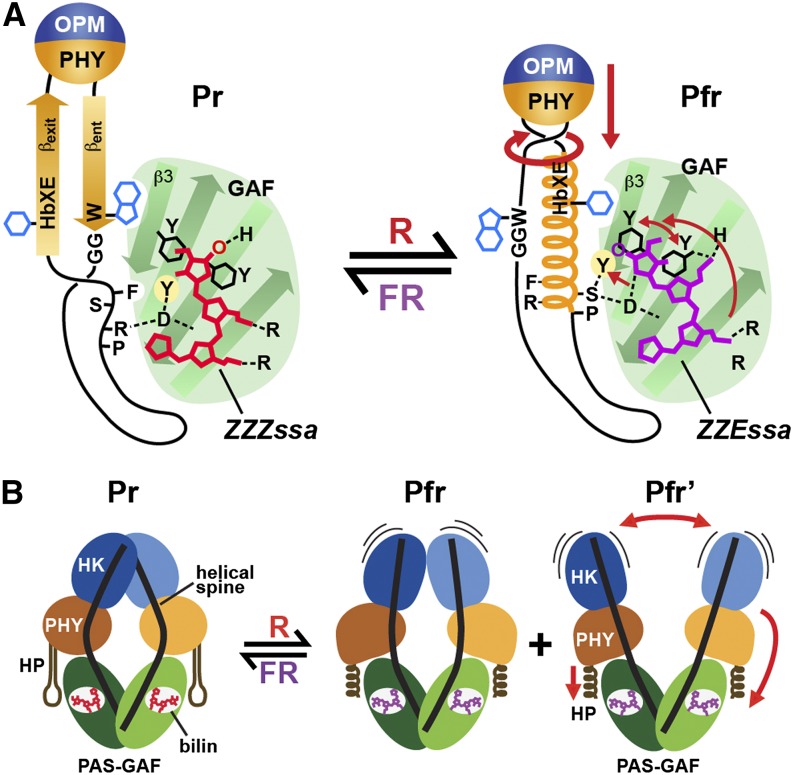
(A) Conformational changes within the GAF domain and hairpin associated with bilin photoisomerization and sliding and hairpin deformation using At-PhyB(Pr) and Pa-BphP(Pfr) as the examples. Upon light-induced rotation of the D pyrrole ring, the bilin breaks its D-ring/His-403 connection and slides (see arrow) within the GAF domain crevice to form a new contact between the D-ring and Asp-307 and the C-ring propionate and His-403. The tryptophan pair, Tyr-276 and Tyr-303 adjacent to the D-ring, rotate in directions opposite to the D-ring rotation. Together, the effects initiate a collision of Tyr-361 with Phe-585, breakage of the Asp-307/Arg-582 contact, and other changes that collectively release the hairpin stem from the GAF domain. The freed hairpin stem becomes helical, swivels, reforms a new contact between Asp-307 and Ser-584 in the PRXSF motif, and swaps the βent for a βexit/Phe-588 connection with the GAF-domain surface. The rotation and helical conversion of strand βexit presumably reorients the PHY domain relative to the GAF domain and/or tugs on the helical spine connecting the PHY domain to OPM (not shown) to eventually actuate signaling changes in the OPM. The conformations of the bilin in the two end states are indicated.
(B) Global conformational changes within the Dr-BphP Phy dimer. The PHY domains adjust their positions relative to their GAF domains through the light-induced conformational changes in the PHY domain hairpin stem from β-strand to helical followed by straightening of the bowed helical spines. Ultimately, the position and/or mobility of the paired OPMs are impacted to alter their signaling potential, in this case phosphotransferase activity by a histidine kinase domain (HK). HP, hairpin. Pfr and Pfr' represent the two photoactivated states observed by SPEM.
([A] is adapted from Burgie et al. [2014a], Figure 5; and [B] from Burgie et al. [2014b], Figure 11.)