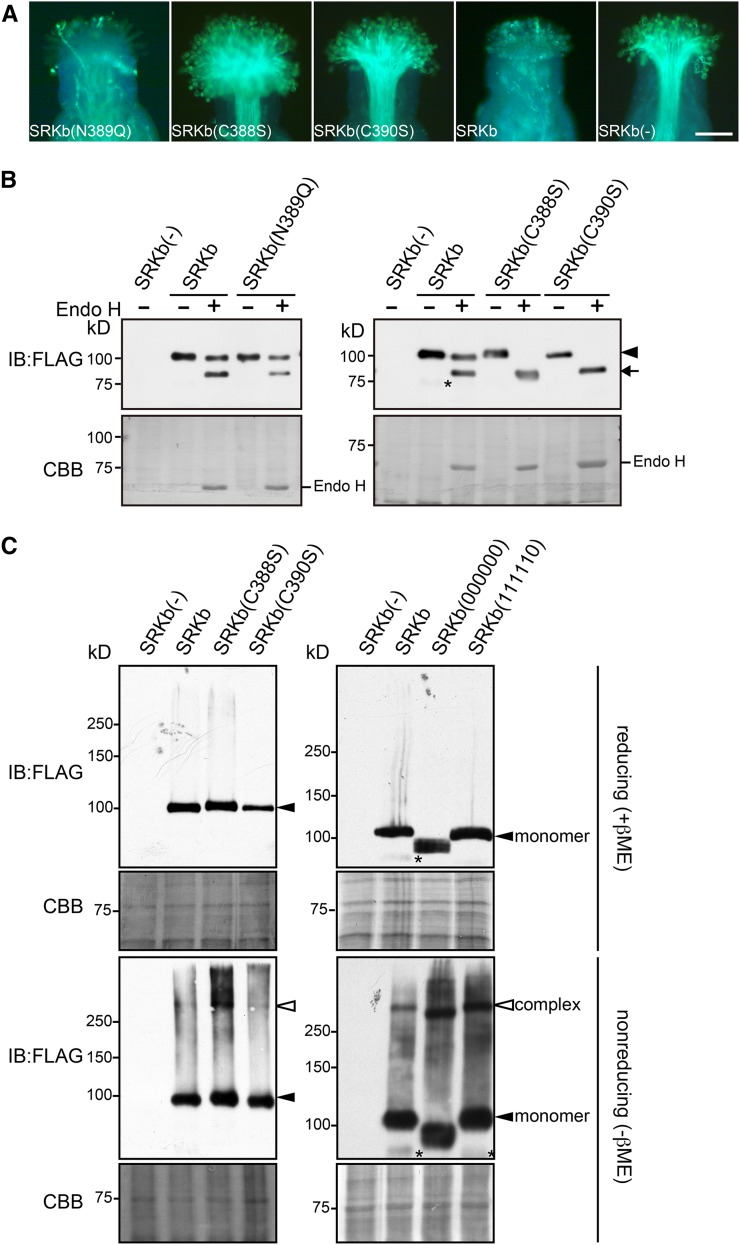
Figure 6.
Functional and Electrophoretic Analysis of SRKb Mutants Carrying Cysteine and N-Glycosylation Motif Mutations in an Essential Region of the SRKb PAN_APPLE Domain.
(A) Microscopic visualization of pollination phenotypes. Representative images are shown for pollination phenotypes observed after pollination with SCRb pollen of stigmas expressing the FLAG-tagged SRKb(N389Q), SRKb(C388S), and SRKb(C390S) mutants, or wild-type SRKb (SRKb), and stigmas from untransformed plants lacking SRKb [SRKb(-)]. Bar = 100 μm.
(B) Sensitivity to Endo H of FLAG-tagged wild-type SRKb, SRKb(N389Q), SRKb(C388S), and SRKb(C390S) proteins. The glycosylated and deglycosylated forms of SRKb proteins are indicated by an arrowhead and arrow, respectively. Sample preparation, enzymatic treatment, and labeling of figure panels were as in Figure 3B. The asterisk indicates a band that likely represents a degradation product of SRKb-FLAG. Note that the SRKb(C388S) and SRKb(C390S) proteins migrate slightly more slowly than wild-type SRKb, likely due to the C-to-S substitution, which has been shown to cause a slight reduction in electrophoretic mobility in other proteins (Sakoh-Nakatogawa et al., 2009).
(C) Detection of disulfide bond-mediated SRKb complexes in stigmas expressing FLAG-tagged wild-type SRKb, SRKb(C388S), SRKb(C390S), SRKb(000000), and SRKb(111110). Total proteins were prepared from flower buds in the presence of 15 mM iodoacetamide, subjected to SDS-PAGE in the presence of β-mercaptoethanol (reducing [+βME]) or in the absence of β-mercaptoethanol (nonreducing [-βME]), followed by immunoblot (IB) analysis with anti-FLAG antibody. Coomassie blue (CBB) staining is shown as loading control. Bands corresponding to SRKb monomers and complexes are indicated. The asterisks show a degradation product of SRKb-FLAG and SRKb(111110).
[See online article for color version of this figure.]