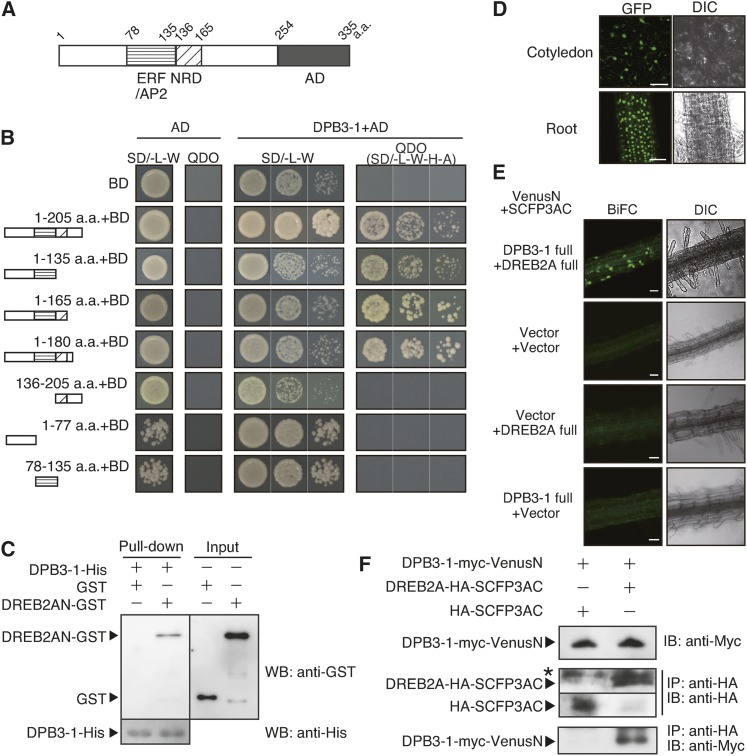
Figure 1.
DPB3-1 Interacts with DREB2A in Yeast Cells, in Vitro and in Vivo.
(A) Schematic diagram of DREB2A. AP2/ERF, DNA binding domain; NRD, negative regulatory domain; AD, activation domain. Numbers indicate the positions of amino acid residues starting from the N terminus.
(B) The growth of yeast cells harboring various truncated forms of DREB2A fused to the GAL4 DNA binding domain (BD). SD/-L-W is the nonselective medium, and SD-L-W-H-Ade (quadruple drop out [QDO]) is the selective medium. DPB3-1 was expressed as a fusion protein with the GAL4 activation domain (AD). a.a., amino acids.
(C) In vitro pull-down assays using GST or a DREB2AN (amino acids 1 to 165 of DREB2A)-GST fusion protein and the DPB3-1-His protein. The fractions pulled down by DPB3-1-His were analyzed with an anti-GST antibody and an anti-His antibody.
(D) Nuclear localization of sGFP-DPB3-1. The cotyledon and root tissues of 35S:sGFP-DPB3-1 plants were observed under a microscope. Confocal images of GFP fluorescence and differential interference contrast (DIC) images are shown. Bars = 100 μm.
(E) Verification of in vivo interaction between DREB2A and DPB3-1 by the BiFC system in transgenic Arabidopsis. The root tissues of the plants expressing DREB2A-SFCP3AC and DPB3-1-VenusN were observed after incubation at 37°C for 2 h. Confocal images of BiFC fluorescence (left) and DIC images (right) are shown. Bars = 50 μm.
(F) Coimmunoprecipitation of DPB3-1-myc-VenusN and DREB2A-HA-SCFP3AC in the transgenic Arabidopsis used in (C) after incubation at 37°C for 2 h. The total protein extracts (upper panel) or immunoprecipitated (IP) fractions using an anti-hemagglutinin (anti-HA) antibody (middle and lower panels) were analyzed by immunoblotting (IB) using anti-HA or anti-myc antibodies. The triangles and the asterisk indicate specific and nonspecific signals, respectively.