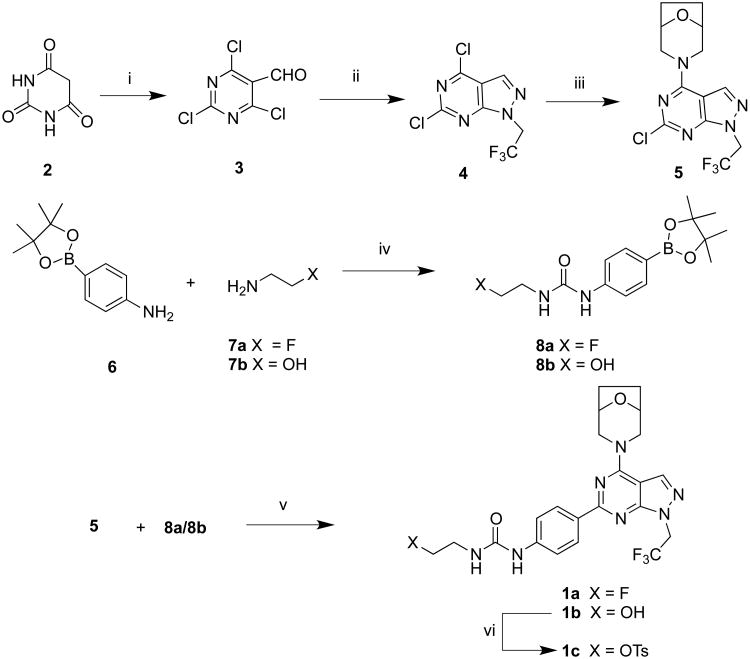
Scheme 1.
Synthesis of ATPFU, hydroxyl and tosyl analogues. i) DMF/POCl3, rt, 72%; ii) CF3CH2NHNH2, EtOH, Et3N, -78 °C, 64%; iii) (1R,5S)8-oxa-3-azabicyclo[3.2.1]octane hydrochloride, Et3N, rt, 60%; iv) triphosgene, CH2Cl2, rt; 84%; v) Na2CO3, acetonitrile, Pd(PPh3)4, 90 °C, 64%; vi) TsCl, CH2Cl2, Et3N, rt, 78%.