Abstract
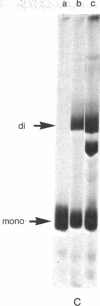
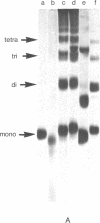
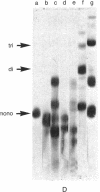
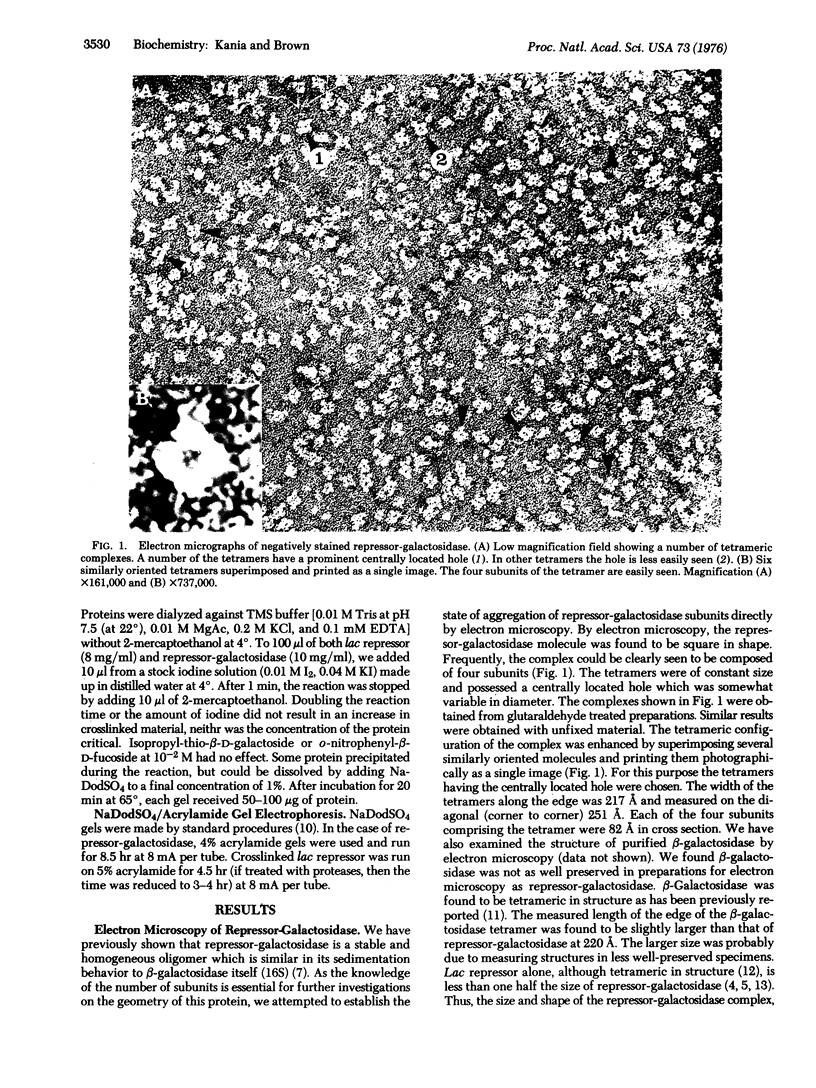
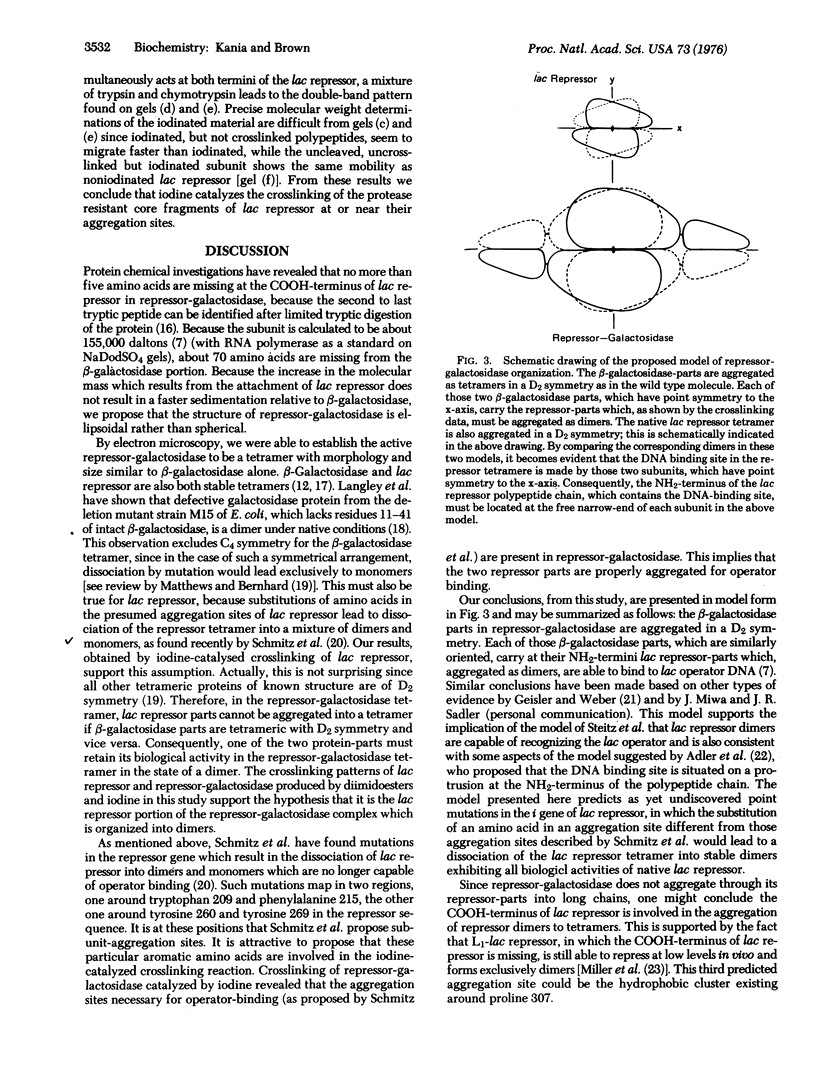
The chimaeric protein repressor-galactosidase, in which fully active lac repressor is covalently linked to the active enzyme beta-galactosidase, was used as a system for probing the quaternary structure of lac repressor. Electron micrographs revealed repressor-galactosidase to be a tetrameric aggregate. When lac repressor, alone, was crosslinked with dimethyl suberimidate, dimers, trimers, tetramers, and oligomers of the protein subunit were produced, whereas crosslinking of the tetrameric repressor-galactosidase resulted in the production of only dimers of the chimaera. Treatment of lac repressor with iodine resulted in the formation of protein dimers; the same result was obtained with repressor-galactosidase. After limited proteolysis of lac repressor, no crosslinking was obtained after treatment with dimethyl suberimidate, whereas iodine still produced a covalent linkage. These results are interpreted as evidence that the lac repressor parts of the tetrameric repressor-galactosidase-chimaera are organized as dimers on the tetrameric-beta-galactosidase core. Because this chimaera has been previously shown to have normal repressor activity [B. Müller-Hill and J. Kania (1974) Nature, 249,561-563], we conclude that lac repressor still is biologically active as a dimeric aggregate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abermann R., Bahl C. P., Marians K. J., Salpeter M. M., Wu R. Studies on the lactose operon. III. Visualization and physical mapping of the lactose repressor-operator complex. J Mol Biol. 1976 Jan 5;100(1):109–114. doi: 10.1016/s0022-2836(76)80038-1. [DOI] [PubMed] [Google Scholar]
- Adler K., Beyreuther K., Fanning E., Geisler N., Gronenborn B., Klemm A., Müller-Hill B., Pfahl M., Schmitz A. How lac repressor binds to DNA. Nature. 1972 Jun 9;237(5354):322–327. doi: 10.1038/237322a0. [DOI] [PubMed] [Google Scholar]
- Beyreuther K., Adler K., Geisler N., Klemm A. The amino-acid sequence of lac repressor. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3576–3580. doi: 10.1073/pnas.70.12.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAVEN G. R., STEERS E., Jr, ANFINSEN C. B. PURIFICATION, COMPOSITION, AND MOLECULAR WEIGHT OF THE BETA-GALACTOSIDASE OF ESCHERICHIA COLI K12. J Biol Chem. 1965 Jun;240:2468–2477. [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning T. G. Iodination of Escherichia coli lac repressor. Effect of tyrosine modification on repressor activity. Biochemistry. 1975 Jun 3;14(11):2512–2520. doi: 10.1021/bi00682a034. [DOI] [PubMed] [Google Scholar]
- Geisler N., Weber K. Isolation of a set of hybrid lac repressors made in vitro between normal lac repressor and its homogeneous tryptic core. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3103–3106. doi: 10.1073/pnas.73.9.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K. E., Villarejo M. R., Fowler A. V., Zamenhof P. J., Zabin I. Molecular basis of beta-galactosidase alpha-complementation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1254–1257. doi: 10.1073/pnas.72.4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Hill B., Kania J. Lac repressor can be fused to beta-galactosidase. Nature. 1974 Jun 7;249(457):561–563. doi: 10.1038/249561a0. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B. Lac repressor and lac operator. Prog Biophys Mol Biol. 1975;30(2-3):227–252. doi: 10.1016/0079-6107(76)90011-0. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Horiuchi T., Yanagida M. Leters to the editors: Structure of the lac repressor studied by negative staining. J Mol Biol. 1975 Feb 5;91(4):515–519. doi: 10.1016/0022-2836(75)90277-6. [DOI] [PubMed] [Google Scholar]
- Platt T., Files J. G., Weber K. Lac repressor. Specific proteolytic destruction of the NH 2 -terminal region and loss of the deoxyribonucleic acid-binding activity. J Biol Chem. 1973 Jan 10;248(1):110–121. [PubMed] [Google Scholar]
- Schmitz A., Schmeissner U., Miller J. H. Mutations affecting the quaternary structure of the lac repressor. J Biol Chem. 1976 Jun 10;251(11):3359–3366. [PubMed] [Google Scholar]
- Steitz T. A., Richmond T. J., Wise D., Engelman D. The lac repressor protein: molecular shape, subunit structure, and proposed model for operator interaction based on structural studies of microcrystals. Proc Natl Acad Sci U S A. 1974 Mar;71(3):593–597. doi: 10.1073/pnas.71.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]