Table 1.
Optimization of reaction conditions: multicomponent versus domino reaction.
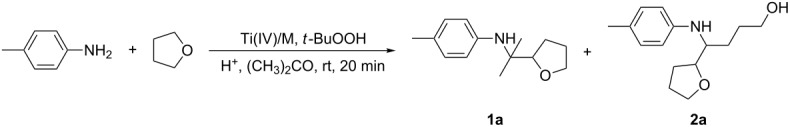 | ||||||
| Entry | TiCl4–M | THF (mL) | Acetone (mL) | CH2Cl2 (mL) | 1 (Yield, %a) | 2 (Yield, %a) |
| 1 | M = Zn | 10 | – | – | – | 84b |
| 2 | M = Zn | 10 | 0.37 (5 mmol) | – | 66 | 11 |
| 3 | M = Zn | –c | 10 | – | 63 | – |
| 4 | M = Zn | –c | 1 | 5 | 70 | – |
| 5 | M = Zn | –c | 0.37 (5 mmol) | 5 | 77 | – |
| 6 | M = Mn | –c | 0.37 (5 mmol) | 5 | 45 | – |
| 7 | M = Fe | –c | 0.37 (5 mmol) | 5 | – | – |
| 8 | – | –c | 0.37 (5 mmol) | 5 | – | – |
| 9 | M = Znd | –c | 0.37 (5 mmol) | 5 | – | – |
aYield determined by 1H NMR spectroscopy with acetophenone added as an internal standard to the crude reaction mixture after work-up. bData taken from [30]. cTHF was added dropwise diluted in a 80 wt % solution of t-BuOOH. dIn the absence of TiCl4.
