Fig. 1.
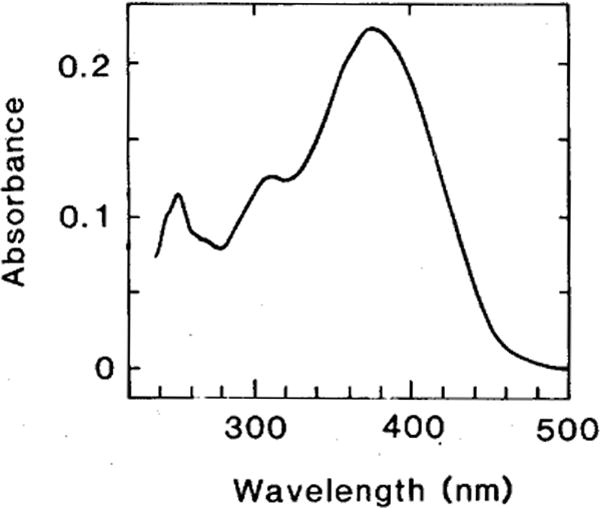
Absorbance spectrum of 11-cis 3,4-didehydroretinal in n-hexane. A purified sample of all-trans 3,4-didehydroretinal in ethanol (127 nmol/ml) was illuminated for 2 h (at 4°C) with a 150 W tungsten bulb. This photoisomerate of the all-trans isomer was injected into the HPLC (column, Ultrasphere; eluant, 12% diethyl ether in n-hexane; flow, 1 ml/min.; see Tsukida et al., ’80 for the sequence of isomer elution) and the 11-cis and other isomers were collected from the HPLC eluant. The spectrum shown in the figure is consistent with the published spectrum of the authentic 11-cis 3,4-didehydroretinal (Schwieter et al., ’62; the spectra of the other isomers were also consistent with published data). When this isomer was reinjected into the HPLC, only a single peak was eluted.
