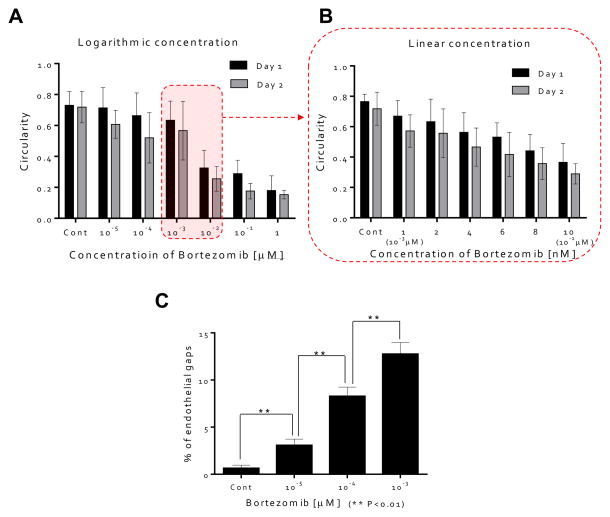
Figure 3. Quantitative analysis of the influence on HUVEC morphology of bortezomib.
(A) Circularities of ECs as a function of bortezomib concentration (0, 10−5, 10−4, 10−3, 10−2, 10−1, and 1 μM). Circularities were > 0.6 for < 10−3 μM), whereas circularities were < 0.4 @ 10−2 μM. (B) Circularities of ECs as a function of bortezomib concentration (0, 1 (10−3 μM), 2, 4, 6, 8, and 10 nM (10−2 μM)). Circularity of the ECs, a sensitive measure of cell health, decreased as the concentration and treatment time increased. (C) HUVECSs were stained with anti-VE-cadherin followed by Alexa 488. Breakdown of VE-cadherin at the doses of bortezomib (10−5, 10−4, & 10−3 μM) was evident through the discontinuity of the green fluorescent line labeling the VE-cadherin junctions compared with intact VE-cadherin junctions in the case of HUVECs cultured in control medium (0 μM). (D) Qualitative analysis of cell junction integrity by measuring the number of gaps observed in the VE-cadherin staining; endothelial gap number increased with increasing concentration of bortezomib at 5 days (0, 10−5, 10−4, and 10−3 μM).