FIG 4.
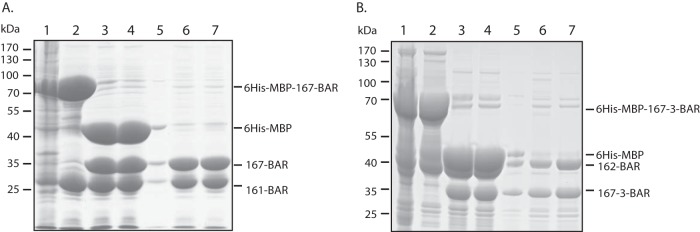
Purification of Rvs161/Rvs167 and Rvs162/Rvs167-3 heterodimers from E. coli. E. coli cells expressing 6His-MBP-RVS167BAR and untagged RVS161BAR (A) or 6His-MBP-RVS167-3BAR and untagged RVS162 (B) were lysed, and the soluble fraction (lane 1) was purified over an amylose column. The eluate (lane 2) was subjected to TEV protease treatment (lane 3) followed by an ultracentrifugation step to separate the soluble fraction (lane 4) from the insoluble fraction (lane 5). The (cleaved) 6His-MBP tag and the 6His-TEV tag were removed from the soluble fraction using a Ni-NTA column. RVS167BAR and RVS161 proteins (A) and RVS167-3BAR and RVS162 proteins (B) were recovered in a 1:1 ratio in the Ni-NTA flowthrough fractions (lanes 6 and 7), indicating copurification due to direct interaction between the BAR domains.
