Abstract
Environmental toxins can promote cardiovascular, metabolic and renal abnormalities, which characterize the cardiorenal metabolic syndrome (CRS). Heavy metals, such as mercury and arsenic, represent two of the most toxic pollutants. Exposure to these toxins is increasing due to increased industrialization throughout much of the world. Studies conducted to understand the impact of environmental toxins have shown a major impact on mitochondrial structure and function. The maladaptive adaptive stress products caused by these toxins, including aggregated proteins, damaged organelles, and intracellular pathogens, can be removed through autophagy, which is also known as mitophagy in mitochondria. Although the underlying mechanisms involved in the regulation of mitophagy in response to pollution are not well understood, accumulating evidence supports a role for maladaptive mitochondrial responses to environmental pollution in the pathogenesis of the CRS. In this review, we discuss ongoing research, which explores the mechanisms by which these toxins promote abnormalities in mitophagy and associated mitochondrial dysfunction and the CRS.
Keywords: Toxins, cardiorenal metabolic syndrome; environmental pollution; mitophagy; mitochondria dysfunction; reactive oxygen species
1. Introduction
The cardiorenal metabolic syndrome (CRS) consists of a constellation of cardiac, renal and metabolic disorders that include insulin resistance, obesity, metabolic dyslipidemia, high blood pressure and evidence of early cardiac and renal disease (Sowers et al. 2011). Factors that contribute to the genesis of metabolic, cardiovascular disease (CVD) and renal abnormalities that characterize the CRS include a genetic predisposition, decreased physical activity and other environmental factors, chronic inflammation, oxidative stress, elevated free fatty acids (FFA), insulin resistance, hyperglycemia, and mitochondrial dysfunction (Jindal, Whaley-Connell, Sowers 2013). There is accumulating evidence that environmental pollution enhances the risk for development of CRS (Colicino et al. 2014). For example, bisphenol A, an essential ingredient in the production of plastic polymers is found in substantial amounts in the urine of 95% of persons in the United States (Hutcheson et al. 2012; Spalding et al. 2009). In animal experiments, increased bisphenol A exposure, even at levels less than those allowed by the US Environmental Protection Agency, has been shown to induce insulin resistance, promote glucose intolerance, and increase adipogenesis (Hutcheson et al. 2012; Spalding et al. 2009). Further, a controlled human exposure study showed that ambient ultrafine particles can cause CVD in people with insulin resistance (Delvin et al. 2014). Additional studies have revealed increased CVD risk after both short- and long-term exposure to high particulate matter air pollution (Hutcheson et al. 2012). Moreover, studies in animal models and humans suggest that long-term exposure to environmental pollutants promotes the development of insulin resistance, hyperglycemia, hypertension, obesity and other metabolic abnormalities through derangement of mitochondrial structure and function (Hutcheson et al. 2012).
Heavy metal pollution due to acidification, industrialization, and the utilization of metals in our industrial operations, is an important growing pollution that has a number of adverse effects on mitochondria and associated metabolic, cardiovascular and renal functions that in turn may contribute to increases in CRS (Han et al. 2013). For example, chronic mercury exposure results in accumulation of this metal in mitochondria, which in turn causes ultrastructural mitochondrial alterations that depolarize the mitochondria membrane and subsequently reduce enzyme activity/adenosine triphosphate (ATP) production and Ca2+ buffering capacity (Atchison and Hare 1994) (Fig.1). The results of a recent study suggest that mitophagy, an autophagy-related pathway specific to mitochondria, has the ability to clear damaged mitochondrial components; thus playing a role in the adaptation of the number and quality of mitochondria to new environmental conditions (Jia and Sowers 2014). However, our understanding of the relationship between environmental pollution, mitophagy regulation, mitochondrial dysfunction, and the development CRS is still in its infancy. The objectives of this review focus on the role of toxins, especially mercury and arsenic, in promoting the abnormal regulation of mitophagy and the development of CRS.
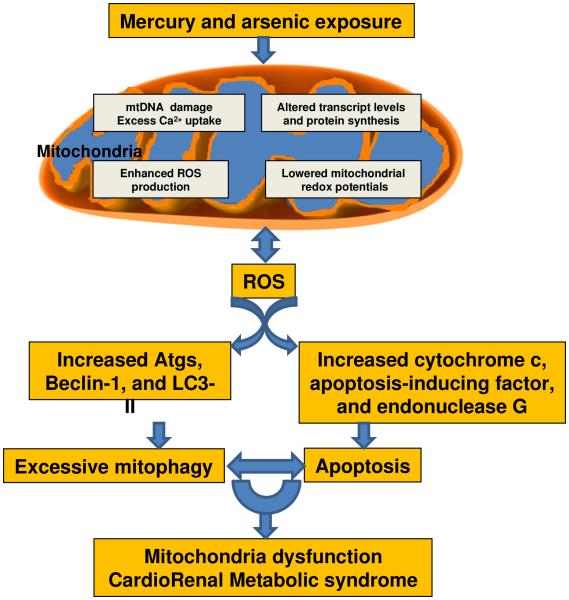
Fig.1.
Proposed roles of mercury and arsenic in the development of mitochondria dysfunction result in CRS. Mercury and arsenic induced mtDNA damage, excess Ca2+ uptake, changes of transcript levels and protein synthesis, as well as ROS production. Increased ROS further exacerbated the impairment of mitochondria and resulted apoptosis and mitophagy in CRS. Abbreviations: mtDNA, mitochondria DNA; ROS, reactive oxygen species; Atgs, autophagy-related genes.
2. Mercury and arsenic exposure affect CRS
Mercury is present in the environment due to natural environmental events or anthropogenic/pollution sources. There are various types of mercury, including elemental mercury vapor, inorganic mercury salts, and organic mercury. Elemental mercury has no significant toxicological effect after ingestion due to poor absorption in the gastrointestinal tract. However, chronic exposure to mercury vapor causes toxic effects, especially on the central nervous system and the kidneys (Eom et al. 2014). Inorganic mercury salts are lipid insoluble and important toxins causing kidney damage (Bridges et al. 2014). Organic mercury, such as methylmercury (MeHg), is the most toxic form of the metal since it is lipid soluble, can be distributed to all tissues, is easily transported across the blood–brain barrier and blood–placenta barrier, and eventually causes central nervous system injury in both animals and humans (Farina et al. 2011). People are often exposed to mercury through industrial sources such as fossil fuel combustion, mining, and incineration plants, as well as from natural sources such as the earth’s crust and volcanoes (Miller et al. 2013). One study found that the blood mercury level in Korea is 3–4 times higher (3.90 μg/L) than in the Unites States (0.83 μg/L), Canada (0.76 μg/L), or Germany (0.58 μg/L), and that these high levels of mercury are associated with increased diabetes mellitus, coronary heart disease, myocardial infarction, stroke, and CVD mortality. Accordingly, it was concluded that chronic exposure to low-dose mercury is significantly associated with a propensity for increases in metabolic, CVD and chronic renal disease (CRD) (Eom et al. 2014).
Arsenic is a widely dispersed element in the earth's crust and exists at an average concentration of approximately 5 mg/kg (Garelick et al. 2008). Arsenic contamination of ground water is found in many countries throughout the world, including the United States. A 2007 study found that over 137 million people in more than 70 countries could be affected by arsenic poisoning of drinking water (Smedley and Kinniburgh 2002). Furthermore, arsenic concentrations have a linear relationship with increasing levels of plasma glucose, plasma lipids, and blood pressure (Spalding et al. 2009). A study performed in the United States, investigated the association of very mild arsenic exposure with the prevalence of type 2 diabetes by measuring urinary arsenic excretion in 788 adults and found that the median urine level of total arsenic was 7.1 μg/L with a prevalence of type II diabetes mellitus of 7.7% and thus concluded that the low levels of exposure to inorganic arsenic may play an important role in increasing the incidence of diabetes (Spalding et al. 2009).
3. Mercury and arsenic exposure promote impairment of mitochondrial function and the CRS
It has been established that mitochondria monitor and evaluate complex information from the environment and intracellular milieu, including the presence of growth factors, reactive oxygen species (ROS), and toxic substances (Kim et al. 2008) (Fig. 1). Mitochondrial dysfunction is recognized as playing a central role in the development of various abnormalities, including disturbed glucose homeostasis, insulin resistance (IR), abdominal fat accumulation, dyslipidemia, hypertension and associated cardiac and renal pathology, all of which characterize the CRS (Whaley-Connell et al. 2011).
3.1. Mercury and arsenic exposure damage mitochondrial structure and function
Our understanding of the mechanisms linking heavy metal exposure with components of the CRS is evolving. Early electrophysiological experiments found that MeHg potently increases intracellular concentrations of Ca2+ and increases the permeability of the plasma membrane to Ca2+, which may cause neurotoxicity (Atchison and Hare 1994). A recent study found that heavy metal pollutants affect the cell function through interference with functional sites in proteins synthesis, displacement of essential elements, and enhancing ROS production, thereby disturbing enzymatic functions (Keunen et al. 2011). For example, at the sub-cellular level, heavy metal pollutants induce the rough endoplasmic reticulum (RER) to undergo a pronounced fragmentation and pathophysiologic changes, such as swelling, cristae reduction, vesiculation, decay of lipid droplets with clusters of electron-dense material at the periphery, marked condensation of nuclear chromatin, and increased number of vesicles of liposomal derivation filled with heterogeneous electron-dense material (Longo et al. 2013). The changes in pathophysiology support the notion that both mercury and arsenic exposure might induce mitochondrial dysfunction, resulting in an increased risk for development of the CRS.
3.2. Mercury and arsenic exposure increase ROS generation
Under physiologic conditions, oxygen consumed in mitochondria is associated with production of oxygen free radicals (ROS) in most cells and tissues. Mitochondrial electron transport generates superoxide (O2·−), an inevitable by-product of complex I and complex III mitochondrial respiration (Smedley and Kinniburgh 2002). Biologically, ROS include O2·−, hydrogen peroxide (H2O2·−), and the hydroxyl radical (OH·). It has been estimated that about 0.2%–2% of the oxygen consumed is converted into O·− by electron transport chain human cells (Aroor et al. 2012). However, the mitochondria of cardiomyocytes have especially high respiratory rates, and approximately 90% of basal cellular ROS in the heart are from mitochondrial production (Demarco et al. 2010). Several enzymatic mechanisms may protect against ROS accumulation in mitochondria. These include conversion of superoxide to H2O2 by superoxide dismutase-2 (SOD2), scavenging of H2O2 by catalase, and the overall antioxidant effects of glutathione peroxidase, and peroxiredoxin III (Sivitz and Yorek 2010).
A number of factors regulate mitochondrial oxidant production, including ROS concentration, mitochondrial antioxidants, electron transport efficiency, nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FAD) H2, uncoupling protein (UCP) activities, cytokines, as well as environment pollution damage (Yang et al. 2007). An excessive exposure of redox-active metals such as mercury and arsenic negatively influences mitochondrial respiration activity, which could be related to their direct potential to increase mitochondrial ROS production via Fenton and Haber-Weiss reactions (Keunen et al. 2011). Increased Ca2+ uptake by mitochondria may also stimulate the generation of ROS (Pieczenik and Neustadt 2007). Therefore, mitochondria undergo several prominent alterations in the early phase of oxidative phosphorylation that may contribute to the impairment of cell function, including increases in production of ROS, loss of mitochondrial membrane potential, and disruption of energy metabolism (Guo et al. 2012) (Fig. 1).
3.3. Mitochondria are targets of ROS
Once overproduction of ROS is induced by heavy metal pollution, these oxidant species can either diffuse out of the mitochondria to mediate signaling functions or induce protein, lipid and DNA damage in the organelle itself (Fig. 1). Studies have shown that ROS can induce a variety of adverse effects, including preferential and sustained mitochondrial DNA (mtDNA) damage, altered mitochondrial transcript levels and mitochondrial protein synthesis, and lowered mitochondrial redox potentials (Yang et al. 2007). mtDNA, a small independent circular genome of 16.5 kb in humans, includes genes encoding for 13 proteins, 22 transfer RNAs (tRNAs), and 2 ribosomal RNAs (rRNAs), which are synthesized by a separate mitochondrial translation system (Byun et al. 2013). These mtDNA can be methylated and are primary sources of ROS generation in response to environment pollution (Yan et al. 2013). Compared to cell nuclear DNA, the sensitivity of mtDNA to oxidative stress-induced damage is much higher due to the lack of chromatin organization and lower DNA repair activity (Keunen et al. 2011). Additionally, the mtDNA is attached to the matrix side of the mitochondrial inner membrane, putting it in close proximity to reactive lipophilic species and reactive lipid oxidation products that are capable of modifying the mtDNA (Yang et al. 2007). Also, ROS mediate post-translational modifications of mitochondrial proteins, which can result in protein inactivation or altered function (Shen 2012). Moreover, this damage occurs prior to or coincidentally with CRS disease development. Thus, the impairment of mitochondria induced by overproduction of ROS may contribute to the pathogenesis of heavy metal exposure-induced CRS.
3.4. Mitochondrial dysfunction is associated with CRS
Mitochondria are essential for intermediary metabolism as well as ATP production, and normally provide more than 90% of the cellular energy (Gao et al. 2008). Mitochondrial dysfunction often occurs in the CRS as evidenced by several observations. First, ROS generated in the mitochondrial respiratory chain have been proposed as intermediate second messengers in the activation of inflammation cytokine expression, such as monocyte chemo-attractive protein (MCP)-1, tumor necrosis factor alpha (TNFα), and interleukin one (IL-1) (Aroor et al. 2013). Second, a decrease in mitochondrial fatty acid oxidation results in increased levels of fatty acyl CoA and diacylglycerol, which, in turn, activate stress related kinase activity and inhibit glucose transport (Rains and Jain 2011). Third, mitochondrial damage in pancreas beta-cells due to increased apoptosis, influx of fatty acids, and activation of stress-related kinases has been reported as secondary mitochondrial dysfunction in type 2 diabetes (Sowers 2013). Fourth, insulin resistance and type 2 diabetes resulting from this secondary mitochondrial dysfunction results in inhibition of hormone-sensitive lipase of adipocytes and decreased endothelial lipoprotein lipase function and the subsequent increase in production of FFA (Kim et al. 2008). The resulting abnormal lipid profile may promote atherosclerosis by impairing the bioavailability of vascular nitric oxide (NO) and associated leukocyte adhesion, inflammation, thrombosis, and vascular smooth muscle cell proliferation (Jindal, Whaley-Connell, Brietzke et al. 2013). Therefore, these abnormalities induced by toxins in the mitochondria are integrally involved in the development of CRS.
4. Mercury and arsenic exposure induce apoptosis and mitophagy
Overproduction of ROS can lead to cell death when mitochondrial anti-oxidative defense and repair systems are overwhelmed (Gul et al. 2012) (Fig. 1). The three types of cell death are apoptosis, necrosis and autophagy. Apoptotic cell death, type 1, is programmed cell death and is characterized by condensation of cytoplasm and chromatin, DNA fragmentation, and cell fragmentation into apoptotic bodies, followed by removal and degradation of the dying cells by phagocytosis (Gozuacik and Kimchi 2004). Autophagy cell death, type 2, is programmed cell death and is characterized by the accumulation of autophagic vesicles such as autophagosomes and autophagolysosomes. This process is often increased when massive cell elimination is demanded or when phagocytes do not have easy access to the dying cells (Liu et al. 2005). One feature that distinguishes apoptosis from autophagic cell death is the source of the lysosomal enzymes used for degradation of dying cells. Apoptotic cells use phagocytic cell lysosomes for this process, whereas cells with autophagic morphology use the dying cells’ endogenous lysosomal machinery in autophagy (Shintani and Klionsky 2004).
4.1. Apoptosis
Programmed cell death is an essential and highly orchestrated process that plays an important role in the development, cellular homeostasis, and prevention of cancer cell growth (Chiarelli and Roccheri 2012). In response to the increased ROS caused by metal pollution toxicity, DNA damage, and cell apoptosis is induced through permeabilization of the outer mitochondrial membrane or the mitochondrial permeability transition pore (mPTP) in the inner mitochondrial membrane (Kubli and Gustafsson 2012). Permeabilization of the outer membrane results in the release of proapoptotic proteins such as cytochrome c, apoptosis-inducing factor (AIF), and endonuclease G (EndoG) to activate apoptosis (Kubli and Gustafsson 2012). In contrast, opening of the mPTP causes rapid influx of solutes and water into the mitochondrial matrix, collapse of the proton gradient, and disruption of ATP synthesis. This influx of solutes and water causes swelling of the inner membrane and eventual rupture of the outer membrane, culminating in necrotic cell death (Kubli and Gustafsson 2012). Both apoptosis and necrotic cell death are increased with the mitochondrial dysfunction that is promoted by heavy metal pollution. Mitochondria are also closely associated with the endoplasmic reticulum (ER), and Ca2+ release from the ER via inositol triphosphate (IP3) receptors is another critical event for the initiation of apoptosis (Sano and Reed 2013). Excess Ca2+ uptake by mitochondria leads to Ca2+ overload and opening of the mPTP (Mei et al. 2013). Bcl-2 family genes are involved in the modulation of cell survival by regulating mitochondrial bioenergetics (Kilbride and Prehn 2013; Kubli and Gustafsson 2012). Another apoptotic gene p53 can translocate to mitochondria, where it promotes mitochondrial membrane permeabilization by interacting with Bcl-2 family proteins and inducing apoptosis (Yi et al. 2011). Thus, both bcl-2 and p53 play important roles in the initiation of apoptosis in response to metabolic stress caused by heavy metal pollution.
4.2. Mitophagy
Both mercury and arsenic induce cell death, not only via apoptosis, but also via autophagy. The autophagy process is regarded as a “mitochondrial quality control” and serves to enhance cell survival through interference with the mitochondrial-mediated apoptotic signals. The role of mitophagy has been tentatively defined as a 'house-cleaning' pathway that eliminates altered mitochondria induced by heavy metal pollution (Quan et al. 2013). Thus, mitophagy is closely coupled to mitochondrial biogenesis. In response to modest mitophagy, cells can utilize their mitochondrial reserve to maintain energy production without affecting function. Indeed, autophagy can remove damaged intracellular macromolecules and organelles and plays a protective role for cell survival (Jiang et al. 2014). Therefore, autophagy not only plays a principal role in the supply of nutrients for cell survival but also plays a constitutive role in cellular homeostasis by acting as a cytoplasmic quality control mechanism to eliminate old or unfolded proteins and damaged organelles (Ma et al. 2013). Up-regulation of the Uth1 and Aup1 genes is early evidence that increases in these gene levels regulate mitophagy by interacting with the autophagy-dedicated protein kinases in yeast (Bhatia-Kissova and Camougrand 2010). Several autophagy-related genes (Atgs) execute and control the autophagic program in human cells. Depending on the environmental stress and cell type, the autophagy program acts as either a survival or death safeguard mechanism (Chiarelli and Roccheri 2012).
Cellular ROS can directly regulate the formation of autophagosomes. Some investigators have shown that ROS regulate autophagy through several different mechanisms, including up-regulation of Beclin-1, oxidation of Atg4 and, causing mitochondrial dysfunction (Chiarelli and Roccheri 2012). For example, Atg4 is subject to oxidation and subsequent inactivation, which leads to accumulation of LC3-II and increased formation of autophagosomes (Kubli and Gustafsson 2012). The PTEN-induced putative kinase 1 (PINK1)/Parkin and E3 ubiquitin ligase parkin signal pathways are important in regulating mitophagy in cells. Parkin promotes ubiquitination of mitochondrial proteins, which serves as a signal for mitophagy (Kubli and Gustafsson 2012). On the other hand, the E3 ubiquitin ligase parkin is predominantly cytosolic under basal conditions but rapidly translocate to mitochondria upon loss of mitochondrial membrane potential in response to pollutant exposure (Kubli and Gustafsson 2012). Thus, mitophagy plays a key role in maintaining mitochondrial function and this process is altered with exposure to environmental toxins.
4.3. Interaction between apoptosis and mitophagy
In general, autophagy is necessary for cell survival under stress through removal of damaged proteins and organelles. However, excessive mitophagy in the absence of increased mitochondrial biogenesis will result in the depletion of mitochondrial bioenergetics reserve and subsequent cell death (Rambold and Lippincott-Schwartz 2011). In cardiomyocytes, overexpression of Beclin1 in the heart amplified the autophagic response to pressure-overload stress, leading to cardiac hypertrophy, cardiac fibrosis, and cardiac dysfunction, suggesting that enhanced autophagic activity by overexpression of Beclin1 may have pathological consequences (Zhu et al. 2007). In coronary vascular cells, overactive autophagy may trigger autophagy-induced cell death resulting in plaque destabilization and is considered a crucial step in the development of myocardial infarction and stroke (Salabei and Conklin 2013). Indeed, when the number of damaged mitochondria exceeds the mitophagy capacity, mitophagy become inactivated, the cell is beyond rescue and apoptosis will become the dominant pathway to minimize extraneous tissue damage upon cell death (Nemchenko et al. 2011). One study found that Atg5 and Atg12 are involved in the initiation of apoptosis in response to diverse stress signals. Indeed, non-conjugated forms of Atg12 and Atg5 contribute to induction of apoptosis, implying that their role in apoptosis may be independent of their role in autophagy (Booth et al. 2014). On the other hand, autophagy may protect cells from apoptosis since Bcl-2 binding to beclin1 inhibits beclin1-mediated autophagy via sequestration of Beclin1 away from class III phosphoinositide kinase PI3K (Ptak et al. 2014). The phosphorylated Bcl-2 could protect cells against apoptosis by preserving the integrity of the mitochondrial outer membrane and by preventing the release of pro-apoptotic proteins into the cytoplasm (Ouyang et al. 2014). Therefore, the interaction between the anti-apoptotic protein Bcl-2 and the autophagy protein Beclin1 may be an important mechanism involved in the regulation of the switch between autophagy and apoptosis.
5. Conclusion
Environmental pollution with heavy metals such as mercury and arsenic can induce the impairment of mitochondrial structure and function, resulting in metabolic, cardiovascular and renal disease, which characterize the CRS. To this point, it appears that mitochondria are the primary target of selective autophagy and that the clearance of dysfunctional mitochondria is important for organelle quality control. Therefore, it is important to gain insights into the mechanisms regulating the balance between survival and death under both normal conditions and environment pollution. The autophagy modulators that regulate mitophagy and mitochondrial integrity may represent future therapeutic targets to address the metabolic, CVD and renal abnormalities that characterize the CRS. Therefore, a better understanding of the mechanisms leading to mitochondrial dysfunction induced by pollution exposure may unmask new strategies to reduce the development of and the morbidity and mortality associated with the CRS.
Acknowledgments
The authors would like to thank Brenda Hunter for her editorial assistance. This research was supported by NIH (R01 HL73101, R01 HL107910) and the Veterans Affairs Merit System (0018) for JRS, and NIH (R01 HL088105) for LAM.
Footnotes
Disclosure Statement The authors have no conflict of interest associated with this manuscript. The authors wish to thank Brenda Hunter for providing editorial help for this manuscript.
References
- 1.Aroor AR, Mandavia C, Ren J, Sowers JR, Pulakat L. Mitochondria and oxidative stress in the cardiorenal metabolic syndrome. Cardiorenal Med. 2012;2:87–109. doi: 10.1159/000335675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62:1543–1552. doi: 10.1016/j.metabol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atchison WD, Hare MF. Mechanisms of methylmercury-induced neurotoxicity. FASEB J. 1994;8:622–629. doi: 10.1096/fasebj.8.9.7516300. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia-Kiššová I, Camougrand N. Mitophagy in yeast: actors and physiological roles. FEMS Yeast Res. 2010;10:1023–1034. doi: 10.1111/j.1567-1364.2010.00659.x. [DOI] [PubMed] [Google Scholar]
- 5.Booth LA, Tavallai S, Hamed HA, Cruickshanks N, Dent P. The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell Signal. 2014;26:549–55. doi: 10.1016/j.cellsig.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bridges CC, Joshee L, Zalups RK. Aging and the disposition and toxicity of mercury in rats. Exp Gerontol. 2014;53:31–39. doi: 10.1016/j.exger.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byun HM, Panni T, Motta V, Hou L, Nordio F, Apostoli P, Bertazzi PA, Baccarelli AA. Effects of airborne pollutants on mitochondrial DNA methylation. Part Fibre Toxicol. 2013;10:18. doi: 10.1186/1743-8977-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiarelli R, Roccheri MC. Heavy metals and metalloids as autophagy inducing agents: focus on cadmium and arsenic. Cells. 2012;1:597–616. doi: 10.3390/cells1030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colicino E, Power MC, Cox DG, Weisskopf MG, Hou L, Alexeeff SE, Sanchez-Guerra M, Vokonas P, Spiro Iii A, Schwartz J, Baccarelli AA. Mitochondrial haplogroups modify the effect of black carbon on age-related cognitive impairment. Environ Health. 2014;13:42. doi: 10.1186/1476-069X-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demarco VG, Whaley-Connell AT, Sowers JR, Habibi J, Dellsperger KC. Contribution of oxidative stress to pulmonary arterial hypertension. World J Cardiol. 2010;2:316–324. doi: 10.4330/wjc.v2.i10.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin RB, Smith CB, Schmitt MT, Rappold AG, Hinderliter A, Graff D, Carraway MS. Controlled exposure of humans with metabolic syndrome to concentrated ultrafine ambient particulate matter causes cardiovascular effects. Toxicol Sci. 2014;140:61–72. doi: 10.1093/toxsci/kfu063. [DOI] [PubMed] [Google Scholar]
- 12.Eom SY, Choi SH, Ahn SJ, Kim DK, Kim DW, Lim JA, Choi BS, Shin HJ, Yun SW, Yoon HJ, Kim YM, Hong YS, Yun YW, Sohn SJ, Kim H, Park KS, Pyo HS, Kim H, Oh SY, Kim J, Lee SA, Ha M, Kwon HJ, Park JD. Reference levels of blood mercury and association with metabolic syndrome in Korean adults. Int Arch Occup Environ Health. 2014;87:501–513. doi: 10.1007/s00420-013-0891-8. [DOI] [PubMed] [Google Scholar]
- 13.Farina M, Rocha JB, Aschner M. Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sci. 2011;89:555–563. doi: 10.1016/j.lfs.2011.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao L, Laude K, Cai H. Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet Clin North Am Small Anim Pract. 2008;38:137–155. doi: 10.1016/j.cvsm.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garelick H, Jones H, Dybowska A, Valsami-Jones E. Arsenic pollution sources. Rev Environ Contam Toxicol. 2008;197:17–60. doi: 10.1007/978-0-387-79284-2_2. [DOI] [PubMed] [Google Scholar]
- 16.Gozuacik D, imchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 17.Gul R, Demarco VG, Sowers JR, Whaley-Connell A, Pulakat L. Regulation of overnutrition-induced cardiac inflammatory mechanisms. Cardiorenal Med. 2012;2:225–233. doi: 10.1159/000339565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo J, Duckles SP, Weiss JH, Li X, Krause DN. 17β-Estradiol prevents cell death and mitochondrial dysfunction by an estrogen receptor-dependent mechanism in astrocytes after oxygen-glucose deprivation/reperfusion. Free Radic Biol Med. 2012;52:2151–2160. doi: 10.1016/j.freeradbiomed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S, Lemire J, Appanna VP, Auger C, Castonguay Z, Appanna VD. How aluminum, an intracellular ROS generator promotes hepatic and neurological diseases: the metabolic tale. Cell Biol Toxicol. 2013;29:75–84. doi: 10.1007/s10565-013-9239-0. [DOI] [PubMed] [Google Scholar]
- 20.Hutcheson R, Rocic P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: the great exploration. Exp Diabetes Res 2012:271028. 2012 doi: 10.1155/2012/271028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia G, Sowers JR. Autophagy: A housekeeper in cardiorenal metabolic health and disease. Biochim Biophys Acta. 20142014 doi: 10.1016/j.bbadis.2014.06.025. doi: 10.1016/j.bbadis.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang Y, Huang W, Wang J, Xu Z, He J, Lin X, Zhou Z, Zhang J. Metformin plays a dual role in MIN6 pancreatic β cell function through AMPK-dependent autophagy. Int J Biol Sci. 2014;10:268–277. doi: 10.7150/ijbs.7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jindal A, Whaley-Connell A, Brietzke S, Sowers JR. Therapy of obese patients with cardiovascular disease. Curr Opin Pharmacol. 2013;13:200–204. doi: 10.1016/j.coph.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jindal A, Whaley-Connell A, Sowers JR. Obesity and heart failure as a mediator of the cerebrorenal interaction. Contrib Nephrol. 2013;179:15–23. doi: 10.1159/000346718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keunen E, Remans T, Bohler S, Vangronsveld J, Cuypers A. Metal-induced oxidative stress and plant mitochondria. Int J Mol Sci. 2011;12(10):6894–6918. doi: 10.3390/ijms12106894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilbride SM, Prehn JH. Central roles of apoptotic proteins in mitochondrial function. Oncogene. 2013;32:2703–2711. doi: 10.1038/onc.2012.348. [DOI] [PubMed] [Google Scholar]
- 27.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Schiff M, Czymmek K, Talloczy Z, Levine B, Dinesh-Kumar SP. Autophagy regulates programmed cell death during the plant innate immune response. Cell. 2005;121:567–577. doi: 10.1016/j.cell.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Longo G, Trovato M, Mazzei V, Ferrante M, Conti GO. Ligia italica (Isopoda, Oniscidea) as bioindicator of mercury pollution of marine rocky coasts. PLoS One. 2013;8(3):e58548. doi: 10.1371/journal.pone.0058548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma T, Zhu J, Chen X, Zha D, Singhal PC, Ding G. High glucose induces autophagy in podocytes. Exp Cell Res. 2013;319:779–789. doi: 10.1016/j.yexcr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mei Y, Thompson MD, Cohen RA, Tong X. Endoplasmic reticulum stress and related pathological processes. J Pharmacol Biomed Anal. 2013;1:1000107. [PMC free article] [PubMed] [Google Scholar]
- 33.Miller S, Pallan S, Gangji AS, Lukic D, Clase CM. Mercury-associated nephrotic syndrome: a case report and systematic review of the literature. Am J Kidney Dis. 2013;62:135–138. doi: 10.1053/j.ajkd.2013.02.372. [DOI] [PubMed] [Google Scholar]
- 34.Nemchenko A, Chiong M, Turer A, Lavandero S, Hill JA. Autophagy as a therapeutic target in cardiovascular disease. J Mol Cell Cardiol. 2011;51:584–593. doi: 10.1016/j.yjmcc.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang C, You J, Xie Z. The interplay between autophagy and apoptosis in the diabetic heart. J Mol Cell Cardiol. 2014;71:71–80. doi: 10.1016/j.yjmcc.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Ptak GE, Toschi P, Fidanza A, Czernik M, Zacchini F, Modlinski JA, Loi P. Autophagy and apoptosis: parent-of-origin genome-dependent mechanisms of cellular self-destruction. Open Biol. 2014;4(6):140027. doi: 10.1098/rsob.140027. doi: 10.1098/rsob.140027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quan W, Jung HS, Lee MS. Role of autophagy in the progression from obesity to diabetes and in the control of energy balance. Arch Pharm Res. 2013;36:223–229. doi: 10.1007/s12272-013-0024-7. [DOI] [PubMed] [Google Scholar]
- 39.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rambold AS, Lippincott-Schwartz J. Mechanisms of mitochondria and autophagy crosstalk. Cell Cycle. 2011;10:4032–4038. doi: 10.4161/cc.10.23.18384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salabei JK, Conklin DJ. Cardiovascular autophagy: crossroads of pathology, pharmacology and toxicology. Cardiovasc Toxicol. 2013;13:220–229. doi: 10.1007/s12012-013-9200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen GX. Mitochondrial dysfunction, oxidative stress and diabetic cardiovascular disorders. Cardiovasc Hematol Disord Drug Targets. 2012;12:106–112. doi: 10.2174/1871529x11202020106. [DOI] [PubMed] [Google Scholar]
- 44.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smedley PL, Kinniburgh DG. A review of the source, behaviour and distribution of arsenic in natural waters. Applied Geochemistry. 2002;17:517–568. DOI: 10.1016/S0883-2927(02)00018-5. [Google Scholar]
- 47.Sowers JR, Whaley-Connell A, Hayden MR. The role of overweight and obesity in the cardiorenal syndrome. Cardiorenal Med. 2011;1:5–12. doi: 10.1159/000322822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sowers JR. Diabetes mellitus and vascular disease. Hypertension. 2013;61:943–947. doi: 10.1161/HYPERTENSIONAHA.111.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spalding A, Kernan J, Lockette W. The metabolic syndrome: a modern plague spread by modern technology. J Clin Hypertens (Greenwich) 2009;11(12):755–60. doi: 10.1111/j.1751-7176.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whaley-Connell A, McCullough PA, Sowers JR. The role of oxidative stress in the metabolic syndrome. Rev Cardiovasc Med. 2011;12:21–29. doi: 10.3909/ricm0555. [DOI] [PubMed] [Google Scholar]
- 51.Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Z, Harrison CM, Chuang GC, Ballinger SW. The role of tobacco smoke induced mitochondrial damage in vascular dysfunction and atherosclerosis. Mutat Res. 2007;621:61–74. doi: 10.1016/j.mrfmmm.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yi CH, Vakifahmetoglu-Norberg H, Yuan J. Integration of apoptosis and metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:375–387. doi: 10.1101/sqb.2011.76.010777. [DOI] [PubMed] [Google Scholar]
- 54.Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]