Abstract
Sterol O-acyltransferase 2 (SOAT2), also known as ACAT2, is the major cholesterol esterifying enzyme in the liver and small intestine (SI). Esterified cholesterol (EC) carried in certain classes of plasma lipoproteins is hydrolyzed by lysosomal acid lipase (LAL) when they are cleared from the circulation. Loss-of-function mutations in LIPA, the gene that encodes LAL, result in Wolman disease (WD) or cholesteryl ester storage disease (CESD). Hepatomegaly and a massive increase in tissue EC levels are hallmark features of both disorders. While these conditions can be corrected with enzyme replacement therapy, the question arose as to what effect the loss of SOAT2 function might have on tissue EC sequestration in LAL-deficient mice. When weaned at 21 days, Lal−/−:Soat2+/+ mice had a whole liver cholesterol content (mg/organ) of 24.7 mg vs. 1.9 mg in Lal+/+:Soat2+/+ littermates, with almost all the excess sterol being esterified. Over the next 31 days, liver cholesterol content in the Lal−/−:Soat2+/+ mice increased to 145 ± 2 mg but to only 29 ± 2 mg in their Lal−/−:Soat2−/− littermates. The level of EC accumulation in the SI of the Lal−/−:Soat2−/− mice was also much less than in their Lal−/−:Soat2+/+ littermates. In addition, there was a >70% reduction in plasma transaminase activities in the Lal−/−:Soat2−/− mice. These studies illustrate how the severity of disease in a mouse model for CESD can be substantially ameliorated by elimination of SOAT2 function.
Keywords: cholesterol esterification, cholesteryl ester storage disease, hepatomegaly, liver transaminase activity, triacylglycerol, unesterified cholesterol
1. Introduction
In mammals, most of the cholesterol present in the major organ systems is unesterified [1]. Exceptions are the adrenal glands and plasma [1,2]. Several organs are capable of generating esterified cholesterol (EC) through the action of either sterol O-acyltransferase 1 (SOAT1) (also known as ACAT1) which is present in steroidogenic tissues, kidneys, sebaceous glands and macrophages, or SOAT2 (ACAT2) which is expressed predominantly in the liver and small intestine [3,4]. The roles that both SOAT1 and SOAT2 play in the formation of cholesteryl esters and the pathogenesis of atherosclerosis have made these enzymes, particularly SOAT2, key targets for pharmacological intervention [5–8].
Atherosclerosis is not the only disease in which tissue EC accumulation is a causative factor. When various classes of lipoproteins such as low density lipoproteins and chylomicron remnants are cleared from the circulation through receptor-mediated and bulk-phase endocytosis, their cholesteryl esters and triacylglycerols are hydrolyzed by lysosomal acid lipase (LAL) [9]. Mutations in LIPA, the gene that encodes LAL, result in either Wolman disease (WD), or cholesteryl ester storage disease (CESD). Whereas WD is a severe, early onset illness caused by complete loss of LAL activity, CESD is a milder, later-onset disease resulting from partial LAL deficiency [10]. Hepatomegaly and a massive increase in tissue EC levels are hallmark features of both disorders. A spontaneous rat model for Wolman disease was described in 1990 [11], and subsequently a mouse model for CESD was generated and characterized [12,13]. These models have been used for the development and testing of an enzyme replacement therapy (ERT) for this disorder [14–16]. This therapy is also being evaluated in humans [17,18].
A recent review summarized the treatment modalities used thus far in patients with CESD [19]. In one case, a statin was used in combination with the cholesterol absorption inhibitor, ezetimibe [20]. We found that in LAL-deficient mice, the addition of ezetimibe to their diet starting at the time they were weaned, resulted in a marked reduction in hepatic EC content and improved liver function [21]. This benefit was attributed primarily to a diminished delivery of intestinally-derived EC to the liver, with a resultant fall in the mass of EC entrapped in the lysosomes. In the current studies we used the CESD mouse model to investigate the extent to which the progression of disease stemming from the absence of LAL might slow in the face of a concurrent loss of SOAT2 function.
2. Materials and methods
2.1 Animals and diets
Lal+/− breeding stock were obtained from the laboratory of Drs. Grabowski and Du at the Children’s Hospital Research Foundation in Cincinnati [12,13]. These mice were of the FVB/N strain and were used to generate Lal+/+ and matching Lal−/− offspring for study at 21 and 93 days of age. In 2003 we purchased Soat2+/− mice (on a BL/6:129S4 background) from the Jackson Laboratory, Bar Harbor, ME. The background was shifted to BL/6:129/Sv over the course of more than eight generations. Lal+/− and Soat2−/− mice were used to generate Lal+/−:Soat2+/− breeding stock that in turn were bred to obtain offspring of the four genotypes (Lal+/+:Soat2+/+, Lal−/−:Soat2+/+, Lal+/+:Soat2−/− and Lal−/−:Soat2−/−) needed for the current studies. Across all litters approximately equal numbers of mice of these four genotypes were obtained, with similar numbers of males and females for each genotype. Only the males were used in the present study. Their age on the day of study averaged 52 ± 1 days. The preceding study with 21- and 93-day old Lal+/+ and Lal−/− mice also used only males. Litters were genotyped at 18–20 days, and weaned at 21 days onto a low-cholesterol, low-fat, rodent chow diet as described [21]. All mice were group-housed in a light-cycled room and were studied in the fed state toward the end of the dark phase of the lighting cycle. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
2.2 Quantitation of total, esterified and unesterified cholesterol in tissues, and liver transaminase activites
After exsanguination of the mice, the liver and entire small intestine were removed, rinsed, blotted and weighed. Aliquots of liver and the whole small intestine were placed in chloroform:methanol (2:1 v/v) for measurement of the esterified (EC) and unesterified cholesterol (UC) fractions [22,23]. All cholesterol quantitation was done using gas chromatography [24]. Plasma total cholesterol concentrations were expressed mg/dl. For the liver and small intestine, the total cholesterol concentration was expressed as mg/g tissue. To obtain whole organ cholesterol content (mg/organ), the total cholesterol concentration was multiplied by the respective whole organ weight. Plasma ALT and AST activities (units/L) were measured by a commercial laboratory.
2.3 Quantitation of liver triacylglycerol content
Hepatic total triacylglycerol concentrations (mg/g) were measured using a method that combines column chromatography and an enzymatic colorimeteric assay [25]. The initial extracts of liver tissue contained [14C]triolein (American Radiolabeled Chemicals, Inc, St Louis, MO) to correct for the procedural losses. These values and liver weight were used to determine whole liver triacylglycerol content (mg/organ).
2.4 Analysis of data
Values are mean ± SEM for the specified number of animals. GraphPad Prism software, version 6.02, (GraphPad, San Diego, CA) was used to perform all statistical analyses. Depending on the design of each experiment, differences between mean values were tested for statistical significance (P < 0.05) by an unpaired two-tailed Student’s t-test, or a one-way analysis of variance.
3. Results
The data in Table 1 demonstrate multiple striking differences between Lal−/− mice and their Lal+/+ littermates, starting at the time of weaning (21 days), and at about 10 weeks later when they were young adults. Genotypic differences in absolute and relative liver weights, already apparent at weaning, were highly pronounced in the 93-day old mice. Comparatively, there were only marginal genotypic differences in small intestine weight at these two age points. The most distinctive feature of the Lal−/− mice was their dramatic increase in hepatic esterified cholesterol (EC) concentrations (mg/g), which, at 93-days, were more than 100-fold higher in the Lal−/− mice vs their Lal+/+ littermates. The increases in both liver mass and hepatic EC concentration combined, resulted in a whole liver cholesterol content (mg/organ) in the Lal−/− mice that was 80-fold more than in the Lal+/+ controls. In the 21-day old mice, the EC concentration in the small intestine of the mutants exceeded that in their wildtype littermates by 8.3-fold. Although the concentrations of EC and UC were not determined in the small intestine of the 93-day old mice, the total content of cholesterol in the intestine of the Lal−/− mice at that age exceeded that in their Lal+/+ littermates by 3.4-fold. In the 93-day-old Lal−/− mice, plasma ALT activities were elevated 20.5-fold compared to their age matched Lal+/+ littermates.
Table 1.
Characteristics of male lysosomal acid lipase-deficient mice at weaning and in early adulthood
| Parameters | Average age (days)
|
|||
|---|---|---|---|---|
| 21±0.1 | 93±1 | |||
| LAL genotype | +/+ | −/− | +/+ | −/− |
| SOAT2 genotype | +/+ | +/+ | +/+ | +/+ |
| Body weight (g) | 10.5±1.5 | 11.6±0.4 | 30.1±0.5 | 28.9±0.8 |
| Liver weight (g) | 0.53±0.09 | 0.77±0.04 | 1.33±0.02 | 4.82±0.18* |
| Relative liver weight (% bw) | 5.0±0.1 | 6.6±0.2* | 4.4±0.1 | 16.8±1.0* |
| Small intestine weight (g) | 0.42±0.05 | 0.62±0.02* | 1.02±0.04 | 1.31±0.10 |
| Hepatic esterified cholesterol concentration (mg/g) | 1.6±0.4 | 28.4±0.4* | 0.4±0.04 | 45.8±2.8* |
| Hepatic unesterified cholesterol concentration (mg/g) | 2.2±0.1 | 2.3±0.2 | 2.0±0.03 | 5.1±0.2* |
| Whole-liver cholesterol content (mg/organ) | 1.9±0.1 | 24.7±0.7* | 3.2±0.1 | 255.6±9.2* |
| Small intestine esterified cholesterol concentration (mg/g) | 0.16±0.02 | 1.34±0.05* | nm | nm |
| Small intestine unesterified cholesterol concentration (mg/g) | 3.0±0.1 | 2.6±0.1 | nm | nm |
| Whole-small intestine cholesterol content (mg/organ) | 1.3±0.1 | 2.5±0.1* | 2.6±0.1 | 8.8±0.4* |
| Plasma ALT activity (units/L) | 34±3 | 48±9 | 37±1 | 759±152* |
All mice were weaned at 21 days of age and thereafter were fed ad libitum a basal rodent chow diet.
They were studied in a fed state.
Values are mean ± 1 SEM of data from 5–7 mice per group.
p<0.05 compared to corresponding Lal+/+:Soat2+/+ mice of the same age.
nm-not measured
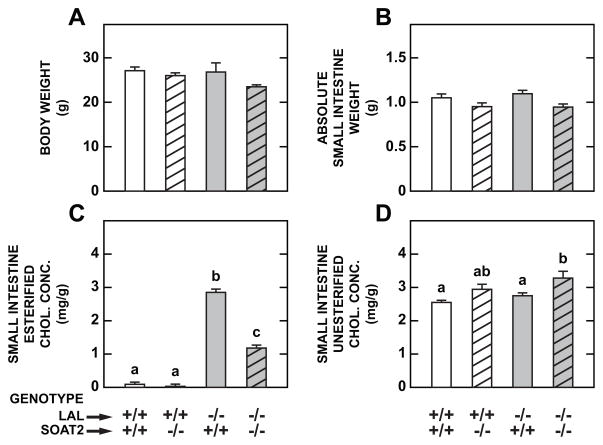
From the data in Table 1, it was clear that even at weaning, there was a substantial buildup of EC in the livers and small intestines of the Lal−/− mice. This progressed to extremely high levels by 93 days of age, with pronounced hepatic dysfunction being evident. Therefore, it was decided that, for the purpose of measuring the impact of SOAT2 deletion on disease progression in the LAL-deficient mice, we would study the Lal−/−:Soat2−/− mice and their wildtype, SOAT2-deficient, and LAL-deficient littermates when they were 52 days old. This age point was about midway between weaning and 93 days of age. As shown in Fig. 1A and 1B, respectively, the final body weights and small intestine weights did not vary significantly amongst the four genotypes. However, there were profound differences in intestinal EC concentrations as a function of genotype (Fig. 1C). Consistent with our previous findings [23], the EC level in the small intestine of wildtype and Soat2−/− mice was very low. In the mice deficient in both LAL and SOAT2, the increment in the intestinal EC concentration was less than half of that seen in their littermates deficient in LAL only. The intestinal UC concentrations changed little with genotype other than a marginal rise in the Lal−/−:Soat2−/− mice (Fig. 1D). Although intestinal TAG levels increase considerably in the LAL-deficient mouse [13], this parameter was not measured in the present study. Plasma total cholesterol concentrations were measured although the data are not illustrated. The values, given as mg/dl, were as follows: Lal+/+:Soat2+/+ (116±6.5), Lal+/+:Soat2−/− (115±6.2), Lal−/−:Soat2+/+ (103±2.2), and Lal−/−:Soat2−/− (101±8.6).
Fig 1.
Loss of the cholesterol-esterifying enzyme SOAT2 in LAL-deficient mice markedly reduced the concentration of esterified cholesterol in the small intestine. Body weight (A), small intestine weight (B), and intestinal concentrations of EC (C) and UC (D) were measured in male mice deficient in either SOAT2, LAL, or both SOAT2 and LAL, at 52 days of age. They were maintained since weaning on a basal rodent chow diet and were in the fed state at the time of study. Values are the mean ± SEM of data from 5 mice in each group except for the Lal−/−:Soat2+/+ mice were n=4. Different letters denote statistically significant values (p < 0.05) as determined by a one-way analysis of variance.
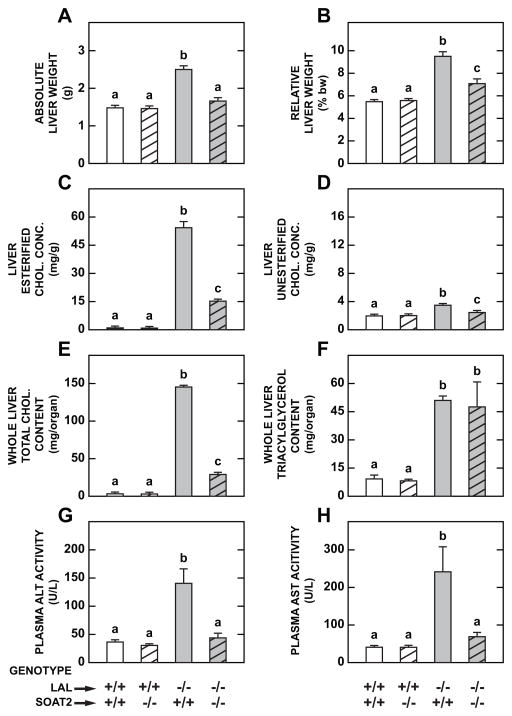
The data for the livers from the same mice that were used for the intestinal measurements are presented in Fig. 2. The deletion of SOAT2 activity in the Lal−/− mice resulted in a marked reduction in the degree of hepatomegaly as shown by the absolute and relative weights for the liver (Fig. 2A and 2B, respectively). There was a dramatic reduction in hepatic EC concentrations in the Lal−/−:Soat2−/− mice vs their Lal−/−:Soat2+/+ littermates (Fig. 2C). In contrast, there were only marginal shifts in the UC concentration in the liver, with the small increase seen in the Lal−/−:Soat2+/+ mice being partially reversed by the loss of SOAT2 activity (Fig. 2D). The most striking change was seen in the data for whole liver total cholesterol content (Fig. 2E). Here, the content in the mice deficient in both LAL and SOAT2 fell to only 20% of that seen in the mice deficient in LAL only. It is important to note that the liver TC content in the 52-day old Lal−/−:Soat2−/− mice (29.0 mg/organ) was essentially about what it was in the LAL-deficient mice at 21-days (24.7 mg) (Table 1). Although the deletion of SOAT2 greatly diminished EC sequestration in the livers of the mice lacking LAL, it had no effect on the content of triacylglycerol in the liver (Fig. 2F). Finally, the profound reduction in liver cholesterol content in the Lal−/−:Soat2−/− mice was accompanied by a decisive improvement in liver function as measured by the plasma activities of ALT (Fig. 2G) and AST (Fig. 2H).
Fig 2.
Loss of SOAT2 activity in LAL-deficient mice results in a dramatic reduction in whole liver cholesterol content that reflects a decrease in liver mass, together with a decisive fall in the esterified cholesterol concentration. Absolute (A) and relative (B) liver weights, concentrations in liver of EC (C) and UC (D), whole liver contents of cholesterol (E) and triacylglycerol (F), and plasma activities of ALT (G) and AST (H) in male mice deficient in either SOAT2, LAL, or both SOAT2 and LAL, at 52 days of age. These data were obtained from the same mice used for the small intestine measurements (Fig. 1). Values are the mean ± SEM of data from 5 mice in each group except for the Lal−/−:Soat2+/+ mice were n=4. Different letters denote statistically significant values (p < 0.05) as determined by a one-way analysis of variance.
4. Discussion
Given the role that SOAT2 plays in generating the esterified cholesterol that is contained in very low density lipoproteins secreted by the liver into the circulation, and in chylomicrons delivered into the lymph from the small intestine [26], it seemed important to investigate the extent to which deletion of SOAT2 might lessen the amount of EC entrapped in the liver and small intestine of the LAL-deficient mouse. The effect was much more dramatic than was anticipated, particularly for the liver. Several of the findings presented here are particularly noteworthy. One of these pertains to the data showing that, even at the time of weaning, the hepatic EC concentration in Lal−/−:Soat2+/+ mice was already elevated almost 18-fold compared to that in Lal+/+:Soat2+/+ littermates. This raises the intriguing question of whether at birth the Lal−/−:Soat2+/+ mice already have a substantial elevation in hepatic EC levels, and if so, what might be found in newborn pups deficient in both LAL and SOAT2. A related question is whether the ablation of SOAT2 function in Lal−/− mice would continue to have a beneficial impact on the liver and small intestine at ages well beyond 52 days, and ultimately on their highly variable lifespan [27].
Another finding warranting comment concerns the lack of change in hepatic TAG levels in the Lal−/−:Soat2−/− mice (Fig. 2F). Here it should be pointed out that, while suppression of SOAT2 activity in a mouse model with dietary cholesterol-associated steatosis enhances hepatic TAG mobilization [28], in that instance the excess TAG is present in cytoplasmic lipid droplets and not sequestered in the lysosomal compartment as it is in LAL deficiency. Studies using enzyme replacement therapy in the CESD mouse model have demonstrated a decisive reduction in hepatic TAG content, even in animals with advanced disease [14,16].
There are many interconnections in cholesterol movement and processing between the small intestine and liver that occur continually [23, 24, 26]. Thus perhaps the most important question raised by these new findings is the extent to which the benefit resulting from global deletion of SOAT2 in LAL deficiency stems from the loss of enzyme activity in the liver versus the small intestine. Studies with liver and small intestine-selective SOAT2 deficient mice have demonstrated that, in both models, there is prevention of diet-induced cholesterol accumulation in the liver and blood [29]. Newly published work using low density lipoprotein receptor-deficient (Ldlr−/−) mice carrying liver or intestine-specific deletion of SOAT2 shows that although EC from both the intestine and liver contribute to the development of atherosclerosis, the Ldlr−/− mice with liver-specific deletion of SOAT2 had less aortic EC accumulation and smaller aortic lesions than the Ldlr−/− mice with intestine-specific SOAT2 deletion [30]. Presumably, the use of LAL-deficient mice with selective deletion of SOAT2 in either the liver or small intestine will establish whether the progression of CESD is driven more by SOAT2 activity in one of these organs than the other. Irrespective of what is determined from such models, we conclude from the current studies that testing of one of the new SOAT2 selective inhibitors [5,8] in this mouse model for CESD might reveal the potential of such agents for the management of this disorder.
Loss of LAL function causes cholesteryl ester storage disease and Wolmans disease
LAL-deficient mice exhibit marked increases in liver mass and cholesterol content
SOAT2 is the major cholesterol esterifying enzyme in liver and small intestine
SOAT2 deletion in Lal−/− mice markedly lowers hepatic and intestinal CE content
Elevation in plasma ALT and AST in Lal−/− mice is prevented when SOAT2 is lost
Acknowledgments
This work was supported entirely by US Public Health Service Grant R01HL009610. We are indebted to Drs. Gregory Grabowski and Hong Du for their gift of LAL heterozygous breeding stock, and to Dr. Lawrence Rudel for helpful discussions regarding recent advances in the pharmacological regulation of SOAT2.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- EC
esterified cholesterol
- ERT
enzyme replacement therapy
- LAL
lysosomal acid lipase
- LIPA
gene that encodes LAL
- NPC1L1
Niemann-Pick C1-Like1
- SI
small intestine
- SOAT2
sterol O-acyltransferase 2
- TAG
triacylglycerol
- TC
total cholesterol
- UC
unesterified cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Adam M. Lopez, Email: adam.lopez@utsouthwestern.edu.
Kenneth S. Posey, Email: kenneth.posey@utsouthwestern.edu.
References
- 1.d’ Hollander F, Chevallier F. Qualitative and quantitative estimation of free and esterified sterols in whole rat and in 23 of its tissues and organs. Biochim Biophys Acta. 1969;176:146–162. [PubMed] [Google Scholar]
- 2.Goodman DS. Cholesterol ester metabolism. Physiol Rev. 1965;45:747–839. doi: 10.1152/physrev.1965.45.4.747. [DOI] [PubMed] [Google Scholar]
- 3.Cases S, Novak S, Zheng YW, Myers HM, Lear SR, Sande E, Welch CB, Lusis AJ, Spencer TA, Krause BR, Erickson SK, Farese RV., Jr ACAT-2, a second mammalian acyl-CoA: cholesterol acyltransferase. Its cloning, expression, and characterization. J Biol Chem. 1998;273:26755–26764. doi: 10.1074/jbc.273.41.26755. [DOI] [PubMed] [Google Scholar]
- 4.Lee RG, Willingham MC, Davis MA, Skinner KA, Rudel LL. Differential expression of ACAT1 and ACAT2 among cells within liver, intestine, kidney, and adrenal of nonhuman primates. J Lipid Res. 2000;41:1991–2001. [PubMed] [Google Scholar]
- 5.Lada AT, Davis M, Kent C, Chapman J, Tomoda H, Omura S, Rudel LL. Identification of ACAT1- and ACAT2-specific inhibitors using a novel, cell-based fluorescence assay: individual ACAT uniqueness. J Lipid Res. 2004;45:378–386. doi: 10.1194/jlr.D300037-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Chang C, Dong R, Miyazaki A, Sakashita N, Zhang Y, Liu J, Guo M, Li BL, Chang TY. Human acyl-CoA: cholesterol acyltransferase (ACAT) and its potential as a target for pharmaceutical intervention against atherosclerosis. Acta Biochim Biophys Sin. 2006;38:151–156. doi: 10.1111/j.1745-7270.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- 7.Farese RV., Jr The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol. 2006;26:1684–1686. doi: 10.1161/01.ATV.0000227511.35456.90. [DOI] [PubMed] [Google Scholar]
- 8.Ohshiro T, Matsuda D, Sakai K, Degirolamo C, Yagyu H, Rudel LL, Omura S, Ishibashi S, Tomoda H. Pyripyropene A, an acyl-coenzyme A: cholesterol acyltransferase 2-selective inhibitor, attenuates hypercholesterolemia and atherosclerosis in murine models of hyperlipidemia. Arterioscler Thromb Vasc Biol. 2011;31:1108–1115. doi: 10.1161/ATVBAHA.111.223552. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein JL, Dana SE, Faust JR, Beaudet AL, Brown MS. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975;250:8487–8495. [PubMed] [Google Scholar]
- 10.Grabowski GA, Du H. Lysosomal Acid Lipase Deficiencies: The Wolman Disease/Cholesteryl Ester Storage Disease Spectrum. In: Valle D, Beaudet A, Vogelstein B, Kinzler K, Antonarakis S, Ballabio A, editors. Scriver’s Online Metabolic and Molecular Bases of Inherited Metabolic Disease. McGraw-Hill; 2012. [Google Scholar]
- 11.Kuriyama M, Yoshida H, Suzuki M, Fujiyama J, Igata A. Lysosomal acid lipase deficiency in rats: lipid analyses and lipase activities in liver and spleen. J Lipid Res. 1990;31:1605–1612. [PubMed] [Google Scholar]
- 12.Du H, Duanmu M, Witte D, Grabowski GA. Targeted disruption of the mouse lysosomal acid lipase gene: long-term survival with massive cholesteryl ester and triglyceride storage. Hum Mol Genet. 1998;7:1347–1354. doi: 10.1093/hmg/7.9.1347. [DOI] [PubMed] [Google Scholar]
- 13.Du H, Heur M, Duanmu M, Grabowski GA, Hui DY, Witte DP, Mishra J. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res. 2001;42:489–500. [PubMed] [Google Scholar]
- 14.Du H, Cameron TL, Garger SJ, Pogue GP, Hamm LA, White E, Hanley KM, Grabowski GA. Wolman disease/cholesteryl ester storage disease: efficacy of plant-produced human lysosomal acid lipase in mice. J Lipid Res. 2008;49:1646–1657. doi: 10.1194/jlr.M700482-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thelwall PE, Smith FE, Leavitt MC, Canty D, Hu W, Hollingsworth KG, Thoma C, Trenell MI, Taylor R, Rutkowski JV, Blamire AM, Quinn AG. Hepatic cholesteryl ester accumulation in lysosomal acid lipase deficiency: non-invasive identification and treatment monitoring by magnetic resonance. J Hepatol. 2013;59:543–549. doi: 10.1016/j.jhep.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Xu YH, Du H, Quinn B, Liou B, Stanton L, Inskeep V, Ran H, Jakubowitz P, Grilliot N, Grabowski GA. Reversal of advanced disease in lysosomal acid lipase deficient mice: A model for lysosomal acid lipase deficiency disease. Mol Genet Metab. 2014;112:229–241. doi: 10.1016/j.ymgme.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Balwani M, Breen C, Enns GM, Deegan PB, Honzik T, Jones S, Kane JP, Malinova V, Sharma R, Stock EO, Valayannopoulos V, Wraith JE, Burg J, Eckert S, Schneider E, Quinn AG. Clinical effect and safety profile of recombinant human lysosomal acid lipase in patients with cholesteryl ester storage disease. Hepatology. 2013;58:950–957. doi: 10.1002/hep.26289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabowski G. Therapy for lysosomal acid lipase deficiency: replacing a missing link. Hepatology. 2013;58:850–852. doi: 10.1002/hep.26366. [DOI] [PubMed] [Google Scholar]
- 19.Reiner Z, Guardamagna O, Nair D, Soran H, Hovingh K, Bertolini S, Jones S, Coric M, Calandra S, Hamilton J, Eagleton T, Ros E. Lysosomal acid lipase deficiency - An under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis. 2014;235:21–30. doi: 10.1016/j.atherosclerosis.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Tadiboyina VT, Liu DM, Miskie BA, Wang J, Hegele RA. Treatment of dyslipidemia with lovastatin and ezetimibe in an adolescent with cholesterol ester storage disease. Lipids Health Dis. 2005;4:26. doi: 10.1186/1476-511X-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuang JC, Lopez AM, Posey KS, Turley SD. Ezetimibe markedly attenuates hepatic cholesterol accumulation and improves liver function in the lysosomal acid lipase-deficient mouse, a model for cholesteryl ester storage disease. Biochem Biophys Res Commun. 2014;443:1073–1077. doi: 10.1016/j.bbrc.2013.12.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beltroy EP, Liu B, Dietschy JM, Turley SD. Lysosomal unesterified cholesterol content correlates with liver cell death in murine Niemann-Pick type C disease. J Lipid Res. 2007;48:869–881. doi: 10.1194/jlr.M600488-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Turley SD, Valasek MA, Repa JJ, Dietschy JM. Multiple mechanisms limit the accumulation of unesterified cholesterol in the small intestine of mice deficient in both ACAT2 and ABCA1. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1012–G1022. doi: 10.1152/ajpgi.00190.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz M, Russell DW, Dietschy JM, Turley SD. Marked reduction in bile acid synthesis in cholesterol 7α-hydroxylase-deficient mice does not lead to diminished tissue cholesterol turnover or to hypercholesterolemia. J Lipid Res. 1998;39:1833–1843. [PubMed] [Google Scholar]
- 25.Beltroy EP, Richardson JA, Horton JD, Turley SD, Dietschy JM. Cholesterol accumulation and liver cell death in mice with Niemann-Pick type C disease. Hepatology. 2005;42:886–893. doi: 10.1002/hep.20868. [DOI] [PubMed] [Google Scholar]
- 26.Rudel LL, Lee RG, Parini P. ACAT2 is a target for treatment of coronary heart disease associated with hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2005;25:1112–1118. doi: 10.1161/01.ATV.0000166548.65753.1e. [DOI] [PubMed] [Google Scholar]
- 27.Aqul A, Lopez AM, Posey KS, Taylor AM, Repa JJ, Burns DK, Turley SD. Hepatic entrapment of esterified cholesterol drives continual expansion of whole body sterol pool in lysosomal acid lipase-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2014 doi: 10.1152/ajpgi.00243.2014. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alger HM, Brown JM, Sawyer JK, Kelley KL, Shah R, Wilson MD, Willingham MC, Rudel LL. Inhibtion of acyl-coenzyme A: Cholesterol acyltransferase 2 (ACAT2) prevents dietary cholesterol associated steatosis by enhancing hepatic triglyceride mobilization. J Biol Chem. 2010;285:14267–14274. doi: 10.1074/jbc.M110.118422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Kelley KL, Marshall SM, Davis MA, Wilson MD, Sawyer JK, Farese RV, Jr, Brown JM, Rudel LL. Tissue-specific knockouts of ACAT2 reveal that intestinal depletion is sufficient to prevent diet-induced cholesterol accumulation in the liver and blood. J Lipid Res. 2012;53:1144–1152. doi: 10.1194/jlr.M024356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Sawyer JK, Marshall S, Kelley KL, Davis M, Wilson MD, Brown JM, Rudel LL. Cholesterol esters (CE) derived from hepatic sterol o-acyltransferase 2 (SOAT2) are associated with more atherosclerosis than CE from intestinal SOAT2. Circ Res. 2014 doi: 10.1161/CIRCRESAHA.115.304378. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]