Abstract
An alpha-zein promoter isolated from maize containing P-box, E motif sequence TGTAAAGT, opaque-2 box and TATA box was studied for its tissue-specific expression in rice. A 1,098 bp promoter region of alpha-zein gene, fused to the upstream of gusA reporter gene was used for transforming rice immature embryos (ASD 16 or IR 64) via the particle bombardment-mediated method. PCR analysis of putative transformants demonstrated the presence of transgenes (the zein promoter, gusA and hpt). Nineteen out of 37 and two out of five events generated from ASD 16 and IR 64 were found to be GUS-positive. A histological staining analysis performed on sections of mature T1 seeds revealed that the GUS expression was limited to the endosperm and not to the pericarp or the endothelial region. GUS expression was observed only in the following seed development stages : milky (14–15 DAF), soft dough (17–18 DAF), hard dough (20–23 DAF), and mature stages (28–30 DAF) of zein-gusA transformed (T0) plants. On the contrary a constitutive expression of GUS was evident in CaMV35S-gusA plants. PCR and Southern blotting analyses on T1 plants demonstrated a stable integration and inheritance of transgene in the subsequent T1 generation. GUS assay on T2 seeds revealed that the expression of gusA gene driven by alpha-zein promoter was stable and tissue-specific over two generations. Results suggest that this alpha-zein promoter could serve as an alternative promoter to drive endosperm-specific expression of transgenes in rice and other cereal transformation experiments.
Keywords: Rice, Particle bombardment, α-zein, GUS, Endosperm, Expression
Introduction
Grains of poaceae grasses (viz., rice, wheat, maize and millets) are used as staple foods across the world. Qualitative and quantitative enhancement of these grains appears to be a major breeding objective of crop improvement world-wide and such an enhancement can be achieved using genetic engineering with ease as compared to traditional crop breeding methods. In general, a genetic enhancement employs transgene(s) which is/are over-expressed using a constitutive promoter(s) with a view to impart desired traits in transgenic plants. In cereals crops, constitutive promoters such as actin (McElroy et al. 1990), CaMV35S (Cauliflower mosaic virus 35S; Odell et al. 1985) or maize ubiquitin (Christensen et al. 1992) are routinely used more as a means to check if the target gene products are expressed throughout the plant system in a routine manner. However, a constitutive expression of transgenes uniformly in most parts in a host plant may not always be required, and a tissue-specific expression would more likely to save the host plant from an imminent and often a huge yield penalty consequent to a mass diversion of the precious resources native to the host plant towards expressing a new target protein unnecessarily throughout the plant (Anami et al. 2013). This particularly is of a major concern when expression of desired traits, as a part of biofortification of the host plant, are sought to be directed to edible parts of the plant (Naqvi et al. 2009). To achieve this, the expression of the transgenes could be regulated at the spatial and temporal level using space (tissue)-specific or time-specific (temporal) promoters. Though use of tissue-specific promoters such as rice glutelin promoter have been reported to be used in rice transformation experiments, such promoters are very few to be used in rice (Vasconcelosa et al. 2003; Takagi et al. 2008). Here, we report isolation and characterization of a maize α-zein promoter that drives an endosperm-specific expression of GUS (β-glucuronidase) reporter gene in rice and the promoter could prove an alternative to the existing endosperm-specific promoters (Stoger et al. 2005) used in most cereal transformation initiatives.
Zeins, the prolamin protein of maize are a family of ethanol soluble proteins found in the maize endosperm. They are synthesized by the membrane-bound polyribosome and get deposited as protein bodies in the endoplasmic reticulum. Synthesis of zeins starts 10–12 days after pollination and continues throughout seed development (Shotwell and Larkins 1989). Zeins are divided into four groups based on their molecular weight namely, α-, β-, γ- and δ-zeins respectively (Coleman and Larkins 1999; Leite et al. 1999). Among different groups, α-zein constitutes the major group and is encoded by more than 65 genes. On the other hand the other classes of zeins are encoded by genes present in few copies (Ueda et al. 1992). The α-zein encoded by large multigene family was further divided into four subfamilies based on the sequence homology as z1A, z1B, z1C and z1D. The 22 kDa α-zein protein is encoded by z1C gene subfamily and the 19 kDa by z1A, z1B and z1D (Miclaus et al. 2011)). The isolation and characterization of the zein genes began in the early 1980s and still goes on. As a result, many zein gene sequences have been deposited in the GenBank database. Though different zein genes has been expressed in several crops like sunflower (Matzke et al. 1984 and Goldsbrough et al. 1986), rice suspension, rice and tobacco protoplast (Dekeyser et al. 1989), Petunia (Williamson et al. 1988), maize (Coleman et al. 1997; Russell and Fromm 1997; Kanobe et al. 2013), tobacco (Schernthaner et al. 1988; Coleman et al. 1996, 2004; Bagga et al. 1995, 1997; Hua et al. 1996; Hoffman et al. 1987), its expression in rice plant was not studied yet. Here we have made an attempt to study the tissue–specific expression of maize 22 kDa α-zein gene’s promoter in rice for the first time.
In the present study, the 22 kDa α-zein gene’s promoter was isolated from maize and fused with the gusA reporter gene. The alpha zein-gusA fusion construct was genetically engineered into rice via particle bombardment-mediated method. Molecular and biochemical analyses of the transformants were carried out to confirm the endosperm-specific expression of zein promoter.
Materials and methods
Promoter isolation
Maize (Zea mays L.) genomic DNA was isolated from maize tissues by CTAB method. The upstream of α-zein gene was amplified using the sequence specific primers, A22F: 5′ CGCAGATCCACTATAATGC 3′ and A22R: 5′ CTATGTTGCTAGTTGGTCTC 3′. The amplified fragment was cloned into the T/A vector pTZ57R/T (Fermentas, USA) and sequenced. The sequence was analyzed using NCBI BLAST (Altschul et al. 1990) to identify related sequences available from the GenBank databases. Multiple sequence alignments were made using CLUSTAL X (1.8). The putative cis elements that involved in the regulation of endosperm-specific expression of the putative promoters were investigated using the PLACE database (Higo et al. 1999).
Transgene construction
The promoter of α-zein gene was fused with the gusA reporter gene coding for the β- glucuronidase (GUS) enzyme in the vector, pCAMBIA 1391Z (CAMBIA, Australia) between BamHI and EcoRI restriction sites (Fig. 1). This construct was designated as PCVZ-3. The above vector harbours hygromycin phospho transferase (hpt) as plant selectable marker gene.
Fig. 1.
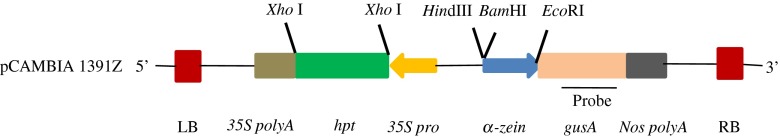
Linear map of pCVZ-3 vector
Rice transformation
Rice transformation was carried out as described by Potrykus et al. (1979). Immature embryos of rice cultivars, ASD16 and IR64 were isolated and pre-cultured on CC medium for 2 days. Pre-cultured immature embryos were transferred to CC osmoticum medium (containing 36.4 g−1 l of each mannitol and sorbitol) 4 h prior to bombardment. Immature embryos were bombarded twice with gold coated plasmid DNA (following the manufacturer’s instruction) using the Gene gun PDS1000/He (Biorad, USA) at 4 h interval. After 4 h of post-treatment on CC osmoticum medium, the bombarded embryos were transferred onto CC medium and incubated in dark. After 2 days of resting, the transformed cells were subjected to selection on CC selection medium containing 30 mg−1 l hygromycin. After two rounds of selection, the transformed calli were transferred onto CC regeneration media (CC medium devoid of 2,4-D) for shoot regeneration and incubated under light for 16 h in a plant growth chamber. The regenerated shoots were transferred onto half strength MS medium for rooting. The fully grown plants were then transferred to soil and maintained in transgenic greenhouse under controlled condition for growth.
PCR analysis
Total genomic DNA was isolated from rice leaf tissue as described by Dellaporta et al. (1983). PCR amplification of zein, gusA, hygromycin and zein-gusA junction sequence was carried out using sequence specific primers A22F: CGCAGATCCACTATAATGC, A22R: CTATGTTGCTAGTTGGTCTC; GUS1F: CAACGAACTGAACTGGCAGA, GUS1R: TTTTTGTCACGCGCTATCAG; HPTF: GCTGTTATGCGGCCATTGGTC, HPTR: GACGTCTGT CGAGAAGTTTG and Z-INTF: ACACCTAGGGAAGCGCACTA, G-INTR: TCTGCCAGTTCAGTTCGTTG respectively.
Southern blot hybridization
Approximately 10 μg of genomic DNA from each sample was digested with the EcoRI, separated on 0.8 % agarose gel and transferred onto a nylon membrane. An internal fragment (PCR amplified) of 878 bp of gusA gene was radioactively labeled using random primer labeling kit (Fermentas, Germany). The radio-labeled probe was purified using probe quant kit G-59 (GE healthcare, USA) following the manufacturers protocol. Purified PCR radiolabeled probe comprising the gusA reporter gene region was used for Southern blot hybridization.
GUS analysis
Histochemical GUS assay was conducted as described by Jefferson (1987). The tissues were incubated overnight at 37 °C in an X-Gluc solution, containing 100 mM sodium phosphate, 1 mM K3Fe(CN)6,1 mM K4Fe(CN)6, 10 mM EDTA, 2 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid cyclohexylammonium salt, 0.1 % Triton X-100 and methanol. After staining, explants were soaked in 70 % ethanol for bleaching. Assayed tissues were observed under a microscope and then photographed. GUS expression was studied in different parts of the transgenic plants and the expression was compared with the constitutive expression of GUS using a transgenic rice line (which was generated in our lab in another experiment) harburing gusA gene driven by CaMV35S promoter.
Histological analysis
The thin sections of GUS stained rice seeds were prepared and the sections were soaked in 70 % ethanol for bleaching. Sections were washed, covered with glycerol and examined microscopically.
Results
Isolation and characterization of 22 kDA α-zein promoter
The 1,098 bp fragment falling to the immediate upstream of ATG of the 22 kDA α-zein gene was isolated from maize genomic DNA (cv. UMI 29) and sequenced. Pairwise alignment with homologous sequences from GenBank revealed 99 % identity with the putative promoter sequence. This ensured the correctness and intactness of the cloned fragment. The 1,098 bp fragent contained transcriptional start site at 1,027 and 68 bp 5′ UTR region. Cis element analysis revealed the presence of basic transcriptional elements such as TATA box. Besides, the occurrence of characteristic endosperm-specific regulatory motifs such as prolamin box and opaque-2 were on expected lines. The cis motifs that fall within the cloned region are summarized in Table 1. The cloned sequence contained the necessary transcriptional elements of zein promoter and 68 bp of the 5′ UTR for expression.
Table 1.
Sequence features identified in the α-zein promoter
| Prolamine box | TGTGTAAAGGT |
| Opaque2box | TCCACATAGA |
| AMYBOX | TATCCAT |
| CAATBOX | CAAT |
| TATABOX | TATAAAT |
Transfer of α-zein promoter-driven gusA gene into rice and confirmation of T0 transgenic rice plants
To study the tissue specificity of α-zein promoter, pCVZ-3 plasmid harbouring gusA gene driven by α-zein promoter and hpt as selectable marker gene was used to transform rice cultivar ASD16 and IR64 by particle bombardment method. Thirty seven events from ASD16 and five events from IR64 were regenerated and transferred to green house. All the transgenic events were morphologically similar to untransformed rice plants and produced fertile flowers.
The presence of zein promoter, gusA and hpt gene sequences in the putative transgenic plants was analyzed. PCR amplification of zein promoter produced a 1,098 bp amplicon while gusA and hpt gene produced expected size of 878 and 630 bp amplicons respectively in all the transgenic events, while in the non-transformed ASD16 and IR64 no amplicon was found. PCR amplification of zein-gusA junction sequence, which was carried out to confirm the integrity of the expression cassette in the T0 plants, resulted in the amplification of a fragment of expected size (875 bp) in five transgenic events subjected to analysis, while no amplicon was seen in the non-transformed ASD16 and IR64 controls (results not shown).
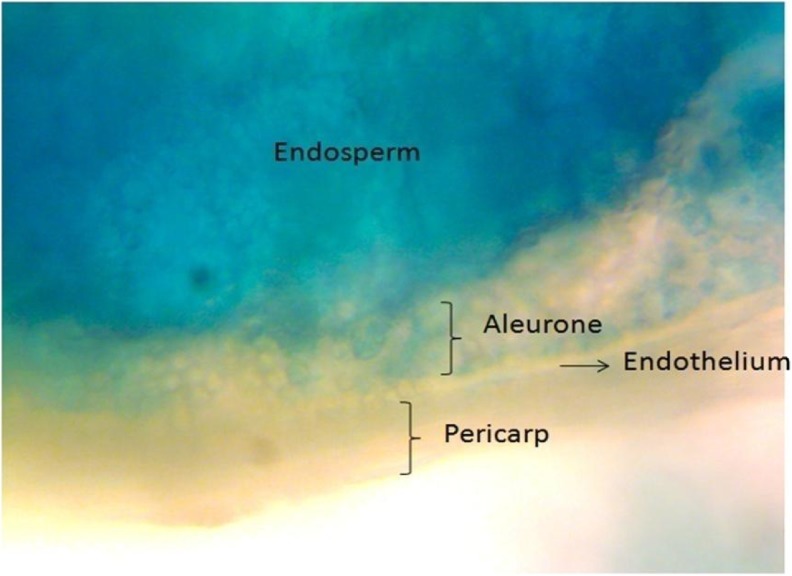
In GUS assay, 19 out of 37 events of ASD16 and two out of five events of IR64 exhibted endosperm-specific GUS expression. No GUS expression was observed in the rest of the 18 ASD and 3 IR64 events. Histological section of X-Gluc stained matured rice seeds revealed that the zein promoter expression was specific to the starch cells with very mild expression in the aleurone layer of the endosperm and not to the pericarp or outer layers of the rice seed (Fig. 2).
Fig. 2.
Histological analysis of X–Gluc stained matured transgenic rice seed expressing zein promoter driven-gusA gene
GUS expression in different parts of transgenic plants
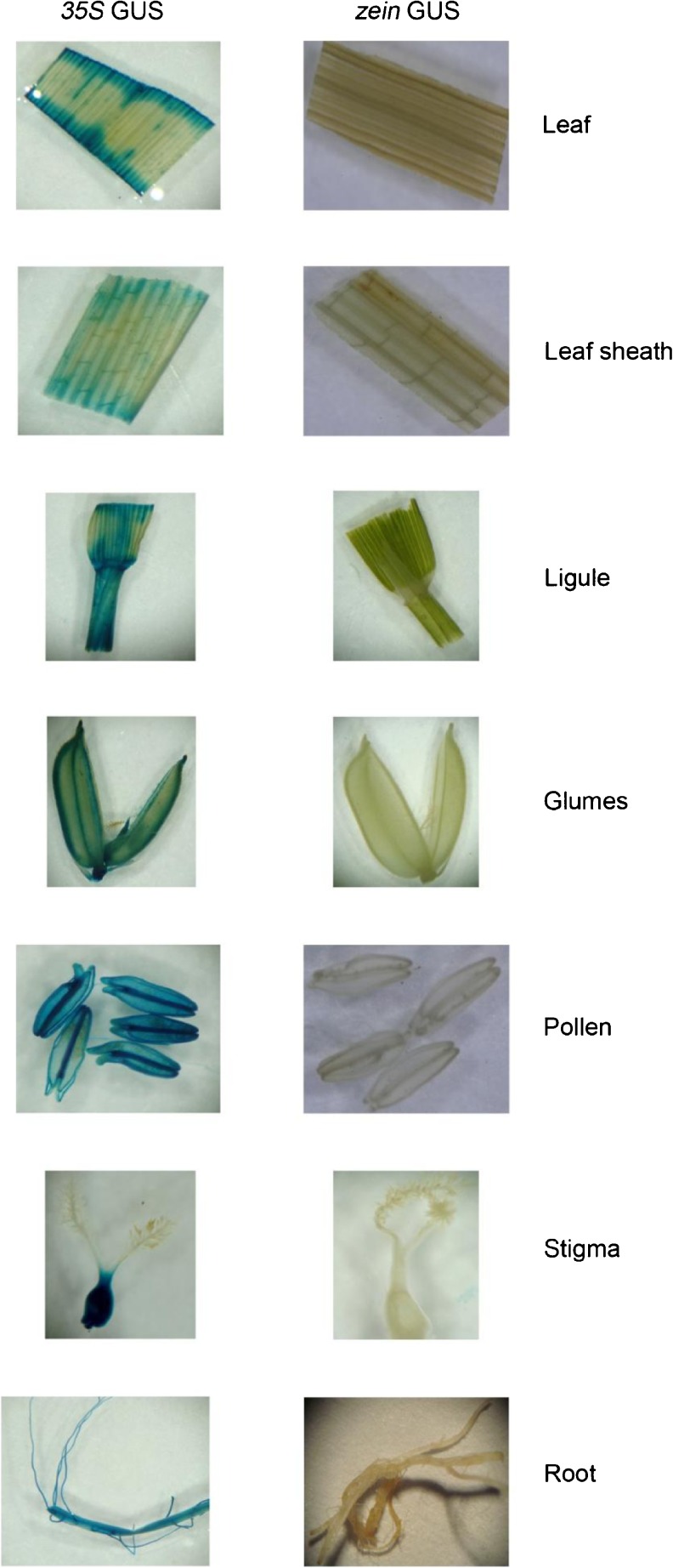
A transgenic homozygous line harbouring gusA gene driven by CaMV35S promoter was used as control. GUS expression was checked in different plant parts from alpha zein-gusA and CaMV35S-gusA transformed plants. In all the 21 events of α-zein-gusA transformed plants, no GUS expression was observed in leaf, leaf sheath, ligules, auricle, glumes, pollen, stigma, embryo, root (Fig. 3), early broom stage (4–10 DAF) of seed development and blue staining was observed only in the endosperm tissue at milky (14–15 DAF), soft dough (17–18 DAF), hard dough (20–23 DAF), and mature stage (28–30 DAF; Fig. 4). All the plant parts assayed showed GUS expression in CaMV35S-gus plants (Figs. 3 and 4).
Fig. 3.
GUS assay in different plant parts of transgenic rice lines
Fig. 4.
Endosperm-specific expression of zein promoter in rice during different stages of seed development (Es-Endosperm, E-embryo)
Transgene inheritance
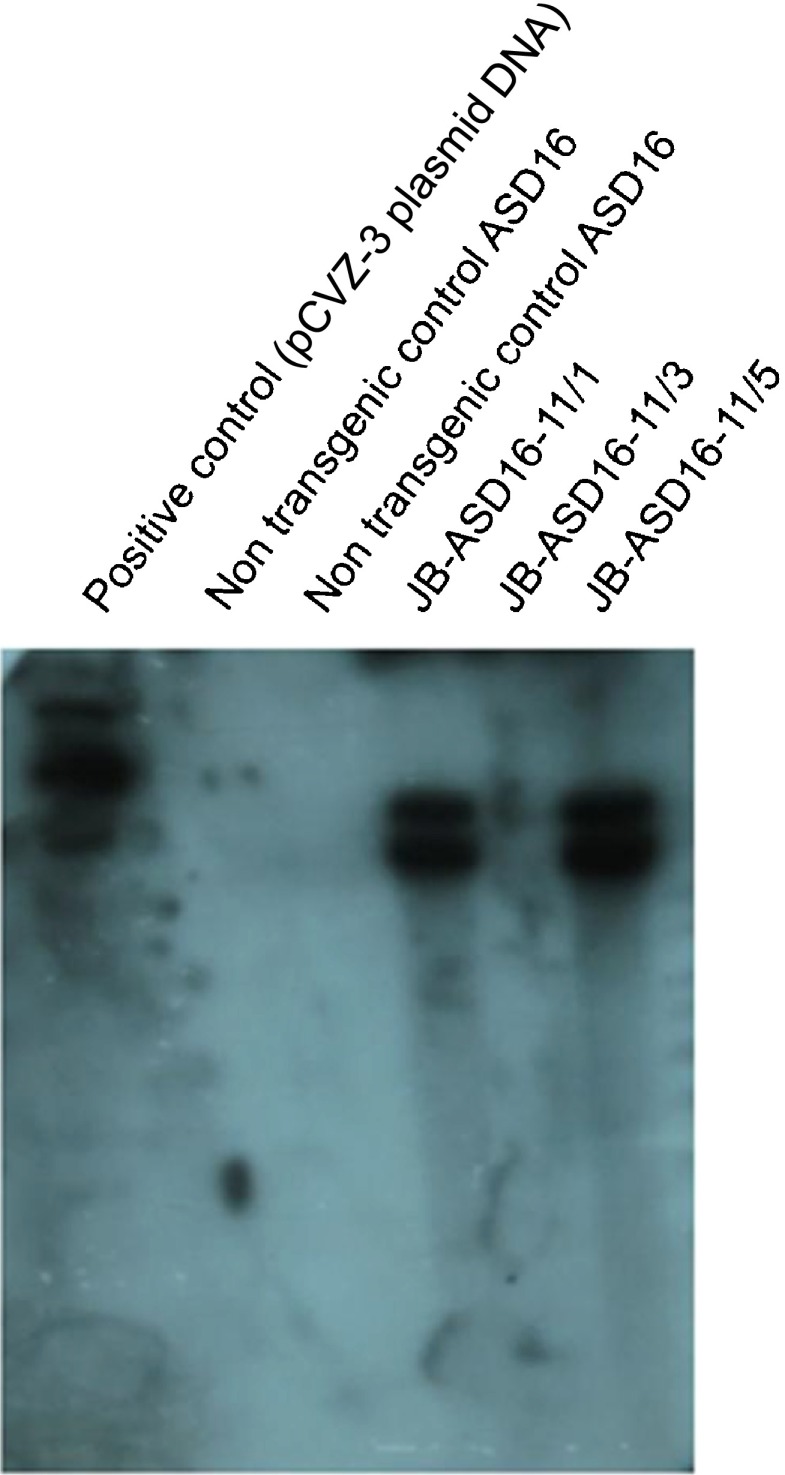
To study the stable inheritance of transgene in subsequent generations, five seeds from JB-ASD16–11 event were subjected to GUS assay and PCR analysis. In GUS assay, performed on a part of the seed (devoid of embryo), expression was observed in four out of five seeds. On germination of remaining part of the seed with embryo, four out of five seeds germinated and three (JB-ASD16–11/1, 11/2, 11/5) were found to be PCR positive for transgene (zein-gusA junction sequence) in PCR analysis. Southern blot hybridization analysis was carried out in the three T1 progenies of event-11 (JB-ASD16–11/1, 11/3, 11/5). PCR positive JB-ASD16–11/2 event produced crooked plant; hence sufficient amount of DNA needed for southern blot hybridization could not be isolated. In Southern blot hybridization analysis of the three T1 progenies of event-11 (JB-ASD16–11/1, 11/3, 11/5), two progenies (JB-ASD16–11/1 and JB-ASD16–11/5) showed hybridization signal (Fig. 5). In T2 generation, the segregation of the transgene was studied in two progenies of an event, JB-ASD16-11/1 and JB-ASD16-11/2. Out of 20 seeds screened, 17 and 12 seeds from JB-ASD16-11/1 and JB-ASD16-11/2 were found to be positive in GUS expression analysis.
Fig. 5.
Southern blot hybridisation analysis of T1 progenies of rice event-11
Discussion
Zein constitutes a major portion of maize kernel protein and is of prolamine type of storage proteins with four major classes namely γ-zein (27 and 16 kDa), α-zein (22 and 19 kDa), β-zein (14 kDa) and δ-zein (10 kDa). The 19 and 22 kDa, α-zein together constitutes 75–85 % of total zein fraction (Shewry and Tatham 1997). In this paper, we have shown that the 22 kDa maize alpha zein promoter is specifically active in the endosperm tissue of transgenic rice plants.
The isolated 1,098 bp fragment of the α-zein gene contained 68 bp of the 5′ UTR and endosperm-specific essential motifs like prolamin box (P-box; 5′-TGTAAAG-3′), opaque-2 box (O2 box; 5′-TCCACATAGA-3′), CAAT box and TATA box that are involved in the regulation of endosperm-specific expression. The interaction between cis-elements in the 5′-flanking regions of seed storage protein genes and seed-specific trans-acting factors regulates the seed-specific expression in these promoters (Bustos et al. 1991 and Chandrasekharan et al. 2003). The interaction between the prolamin box (P-box, AAAG, or CTTT) and endosperm-specific transcription factors, such as zinc-finger protein and the basic leucine zipper (bZIP) transcriptional activator, coordinate the activation of zein gene expression during endosperm development stage (Vicente-Carbajosa et al. 1997). The P-box (5′-TGTAAAG-3′) was identified at −266 region relative to the transcription start site and lies just 22 nucleotides upstream of the binding site for O2. Schmidt et al. (1992) suggested that O2 may interact with factors binding the P-box to activate 22-kDa zein gene expression. It was reported that 7 bp P-box sequence was common in cereals like oats (Shotwell et al. 1990), wheat (Colot et al. 1987), barley (Marris et al. 1988), and sorghum (DeRose et al. 1989) and plays a key role in regulating the endosperm-specific expression.
Reporter genes help in analyzing the tissue-specific expression of promoters and among the different reporter gene systems, gusA gene was widely used because of its sensitivity and simplicity. The reporter gene, gusA was placed under the control of the α-zein promoter and attempts were made to demonstrate the endosperm-specific activity of the α-zein promoter by transforming rice with zein-gusA construct. The GUS activity driven by α-zein promoter was first detected in seed endosperm at 14 DAF (milky stage) and continued till seed maturity (30 DAF). This was similar to the onset of zein synthesis (from 12 DAF) in maize endosperm (Larkins et al. 1984) and therefore, both the temporal specificity and tissue specificity of zein promoter in heterologous system resembles the activity of zein promoters in maize.
Constitutive expression and endosperm-specific expression was compared to demonstrate the spatial specificity of α-zein promoter. Strong GUS expression was observed in all the plant parts such as leaf, leaf sheath, ligule, glumes, pollen, stigma, roots and different stages of seed development till maturity in a homozygous CaMV35S-gus line while in 21 zein-gusA lines the GUS expression was observed only in the endosperm of seeds and not in other plant parts. In zein-gusA lines GUS accumulation began from 14 DAF (milky stage) and continued till maturity (30 DAF) coinciding with the zein synthesis in the endosperm tissue. Due to increased accumulation of beta-glucuronidase with time in the seed endosperm, the intensity of the blue colour in GUS assay increased. Russell and Fromm (1997) found that the ZmZ27 (γ-zein) gene expression was constantly increasing from 14 to 26 DAF in transgenic maize seeds which was consistent to the endogenous γ-zein mRNA levels (Prioul et al. 1994 and Pe’rez-Grau et al. 1986).
The histological analysis of GUS stained matured seed sections revealed that the GUS expression was localized only to the starch and aleurone cells of the seed endosperm portion and not in the pericarp and endothelium cells. In an earlier report, Schernthaner et al. (1988) expressed maize 23 kDa zein promoter in dicot plant and were able to observe endosperm-specific GUS expression in tobacco seeds. Recently it was found that in addition to zein strong expression in the endosperm starch cells, it was also active in the aleurone layer (Reyes et al. 2011). GUS analysis study in T1 and T2 transgenic rice seeds showed stable inheritance and expression of α-zein promoter over generations. Stable integration and inheritance was further demonstrated by Southern blot analysis which detected two sites of transgene integration in the event JB-ASD-16/11. These results suggest that the 1,098 bp fragment of the zein gene is sufficient to drive stable endosperm-specific GUS expression in monocots.
Tissue-specific expression of seed protein genes from legumes (Beachy et al. 1985; Sengupta-Gopalan et al. 1985; Chen et al. 1986; Okamuro et al. 1986), wheat (Colot et al. 1987), Rice (Wu et al. 1998; Patti et al. 2012) and maize (Schernthaner et al. 1988; Russell and Fromm 1997) have been shown previously. The zein promoter consisting 933 bp of 23 kDa zein gene and 1,100 bp of 27 kDa zein gene was expressed in tobacco (Schernthaner et al. 1988) and maize (Russell and Fromm 1997) and endosperm-specific expression was achieved by them. But in 3.1 kb zein4 transformed sunflower cells, the 3.1 kb zein4 gene fragment was transcribed but no functional zein protein was detected (Matzke et al. 1984). The expression of zein promoter in protoplast cells was much weak and in rice protoplast, the expression of 700 bp zein4 was 100 fold less compared to 2′ octopine promoter (Dekeyser et al. 1989).
Until now, glutelin promoter was widely used for the endosperm-specific expression of foreign genes in rice, especially in plant molecular pharming and biofortification programmes (Vasconcelosa et al. 2003; Takagi et al. 2008). For the first time we report the stable and tissue-specific expression of maize alpha zein promoter in rice. As an alternative to rice glutellin promoter, maize alpha zein promoter can be successfully used for endosperm-specific expression in rice transformation experiments. Our results suggest that the 1,098 bp promoter of zein gene (containing P-box, which contains a highly conserved endosperm or E motif (Shewry and Halford 2002) sequence TGTAAAGT, opaque-2 box (O’Shea et al. 1989) and TATA box) is sufficient for driving the endosperm-specific expression of cloned genes and can be used for expression of foreign genes that demands specificity in plant transformation experiments. Further results are suggestive that this α-zein promoter could prove to be a good alternative to the traditional/constitutive promoter for driving an endosperm-specific expression of transgene in rice and possibly in other cereals too at least in the experimental attempts to achieve an endosperm-specific expression with a view to avoiding a yield penalty.
Acknowledgments
We are grateful to the Department of Biotechnology (DBT), New Delhi for providing financial support for this work.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Anami S, Njuguna E, Coussens G, Aesaert S, van Lijsebettens M. Higher plant transformation: principles and molecular tools. Int J Dev Biol. 2013;57:483–494. doi: 10.1387/ijdb.130232mv. [DOI] [PubMed] [Google Scholar]
- Bagga S, Adams H, Kemp JD, Sengupta-Gopalan C. Accumulation of 15-kilodalton zein in novel protein bodies in transgenic tobacco. Plant Physiol. 1995;107:13–23. doi: 10.1104/pp.107.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagga S, Adams HP, Rodriguez FD, Kemp JD, Sengupta-Gopalan C. Coexpression of the maize δ-zein and β-zein genes results in stable accumulation of δ-zein in endoplasmic reticulum-derived protein bodies formed by β-zein. Plant Cell. 1997;9:1683–1696. doi: 10.1105/tpc.9.9.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy RN, Chen ZL, Horsch RB, Rogers SG, Hoffman NJ, Fraley RT. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985;4:3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustos M, Begum D, Kalkan F, Battraw M, Hall TC. Positive and negative cis-acting DNA domains are required for spatial and temporal regulation of gene expression by a seed storage protein promoter. EMBO J. 1991;10:1469–1479. doi: 10.1002/j.1460-2075.1991.tb07667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan MB, Bishop KJ, Hall TC. Module-specific regulation of the β-phaseolin promoter during embryogenesis. Plant J. 2003;33:853–866. doi: 10.1046/j.1365-313X.2003.01678.x. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Schuler MA, Beachy RN. Functional analysis of regulatory elements in a plant embryo-specific gene. Proc Natl Acad Sci U S A. 1986;83:8560–8564. doi: 10.1073/pnas.83.22.8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol. 1992;18:675–689. doi: 10.1007/BF00020010. [DOI] [PubMed] [Google Scholar]
- Coleman CE, Larkins BA. The prolamins of maize. In: Shewry PR, Casey R, editors. Seed proteins. London: Kluwer Academic Press; 1999. pp. 109–139. [Google Scholar]
- Coleman CE, Herman EM, Takasaki K, Larkins BA. The maize γ-zein sequesters α-zein and stabilizes its accumulation in protein bodies in transgenic tobacco endosperm. Plant Cell. 1996;8:2335–2345. doi: 10.1105/tpc.8.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CE, Clore AM, Ranch JP, Higgins R, Lopes MA, Larkins BA. Expression of a mutant α-zein creates the floury2 phenotype in transgenic maize. Proc Natl Acad Sci U S A. 1997;94:7094–7097. doi: 10.1073/pnas.94.13.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CE, Yoho PR, Escobar S, Ogawa M. The accumulation of α-zein in transgenic tobacco endosperm is stabilized by co-expression of β-zein. Plant Cell Physiol. 2004;45(7):864–871. doi: 10.1093/pcp/pch104. [DOI] [PubMed] [Google Scholar]
- Colot V, Robert LS, Kavanagh TA, Bevan MW, Thompson RD. Localization of sequences in wheat endosperm protein genes which confer tissue-specific expression in tobacco. EMBO J. 1987;6:3559–3564. doi: 10.1002/j.1460-2075.1987.tb02685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekeyser R, Claes B, Marichal M, Montagu MV, Caplan A. Evaluation of selectable markers for rice transformation. Plant Physiol. 1989;90:217–223. doi: 10.1104/pp.90.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version 2. Plant Mol Biol Report. 1983;1:19–22. doi: 10.1007/BF02712670. [DOI] [Google Scholar]
- DeRose RT, Ma DP, Kwon IS, Hasnain SE, Klassy RC, Hall TC. Characterization of the Kafirin gene family from sorghum reveals extensive homology with zein from maize. Plant Mol Biol. 1989;12:245–256. doi: 10.1007/BF00043202. [DOI] [PubMed] [Google Scholar]
- Goldsbrough PB, Gelvin SB, Larkins BA. Expression of maize zein genes in transformed sunflower cells. Mol Gen Genet. 1986;202:374–381. doi: 10.1007/BF00333265. [DOI] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res. 1999;27:297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman LM, Donaldsont DD, Bookland R, Rashka K, Herman EM. Synthesis and protein body deposition of maize 15-kd zein in transgenic tobacco seeds. EMBO J. 1987;6(11):3213–3221. doi: 10.1002/j.1460-2075.1987.tb02638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua L, Chonglie M, Qian Z, Guangming A. Amplification of maize zein gene promoter by PCR and it drives GUS gene expression in transgenic tobacco seeds. Chin J Biotechnol. 1996;12(3):295–300. [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Report. 1987;5:387–405. doi: 10.1007/BF02667740. [DOI] [Google Scholar]
- Kanobe MN, Rodermel SR, Bailey T, Scott MP. Changes in endogenous gene transcript and protein levels in maize plants expressing the soybean ferritin transgene. Front Plant Sci. 2013;4(196):1. doi: 10.3389/fpls.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B, Pedersen K, Marks M, Wilson D. The zein proteins of maize endosperm. Trends Biochem Sci. 1984;9:306–308. doi: 10.1016/0968-0004(84)90297-4. [DOI] [Google Scholar]
- Leite A, Neto GC, Vettore AL, Yunes JA, Arruda P. The prolamins of sorghum, coix and millets. In: Shewry PR, Casey R, editors. Seed proteins. London: Kluwer Academic Press; 1999. pp. 141–157. [Google Scholar]
- Marris C, Gallois P, Kreis M. The 5′-flanking region of a barley B hordein gene controls tissue and developmental specific CAT expression in tobacco plants. Plant Mol Biol. 1988;10:359–366. doi: 10.1007/BF00029886. [DOI] [PubMed] [Google Scholar]
- Matzke MA, Susani M, Binns AN, Lewis ED, Rubenstein I, Matzke AJM. Transcription of a zein gene introduced into sunflower using a Ti plasmid vector. EMBO J. 1984;3(7):1525–1531. doi: 10.1002/j.1460-2075.1984.tb02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy D, Zhang W, Cao J, Wu R. Isolation of an efficient actin promoter for use in rice transformation. Plant Cell. 1990;2:163–171. doi: 10.1105/tpc.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miclaus M, Xu JH, Messing J. Differential gene expression and epiregulation of alpha zein gene copies in maize haplotypes. PLoS Genet. 2011;7(6):e1002131. doi: 10.1371/journal.pgen.1002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi S, Zhu C, Farre G, Ramessar K, Bassie L, Breitenbach J, Perez-Conesa D, Ros G, Sandmann G, Capell T, Christou P. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci U S A. 2009;106:7762–7767. doi: 10.1073/pnas.0901412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea EK, Rheba R, Stafford WF, Kim PS. Preferential heterodimer formation by isolated leucine zippers from fos and jun. Science. 1989;245:646–648. doi: 10.1126/science.2503872. [DOI] [PubMed] [Google Scholar]
- Odell JT, Nagy F, Chua NH. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature. 1985;313:810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Okamuro JK, Jofuku KD, Goldberg RB. Soybean seed lectin gene and flanking non seed protein genes are developmentally regulated in transformed tobacco plants. Proc Natl Acad Sci U S A. 1986;83:8240–8244. doi: 10.1073/pnas.83.21.8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti T, Bembi B, Cristin P, Mazzarol F, Secco E, Pappalardo C, Musetti R, Martinuzzi M, Versolatto S, Cariati R, Dardis A, Marchetti S. Endosperm-specific epression of human acid beta-glucosidase in a way rice. Rice. 2012;34(5):1–15. doi: 10.1186/1939-8433-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe’rez-Grau L, Cordatas J, Puigdome’nech P, Palau J. Accumulation and subcellular localization of glutelin-2 transcripts during maturation of maize endosperm. FEBS Lett. 1986;202:145–148. doi: 10.1016/0014-5793(86)80666-4. [DOI] [Google Scholar]
- Potrykus I, Harms CT, Lorz H. Callus formation from cell culture protoplasts of corn(Zea mays L.) Theor Appl Genet. 1979;54:209–214. doi: 10.1007/BF00267709. [DOI] [PubMed] [Google Scholar]
- Prioul JL, Jeannette E, Reyss A, Gre’gory N, Giroux M, Hannah LC, Causse M. Expression of ADPglucose pyrophosphorylase in maize (Zea mays L.) grain and source leaf during grain filling. Plant Physiol. 1994;104:179–187. doi: 10.1104/pp.104.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes FC, Chung T, Holding D, Jung R, Vierstra R, Otegui MS. Delivery of prolamins to the protein storage vacuole in maize aleurone cells. Plant Cell. 2011;23:769–784. doi: 10.1105/tpc.110.082156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DA, Fromm ME. Tissue-specific expression in transgenic maize of four endosperm promoters from maize and rice. Transgenic Res. 1997;6:157–168. doi: 10.1023/A:1018429821858. [DOI] [PubMed] [Google Scholar]
- Schernthaner JP, Matzke MA, Matzke AJM. Endosperm-specific activity of a zein gene promoter in transgenic tobacco plants. EMBO J. 1988;7(5):1249–1255. doi: 10.1002/j.1460-2075.1988.tb02938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Ketudat M, Aukerman MJ, Hoschek G. Opaque-2 is a transcriptional activator that recognizes a specific target site in 22-kD zein genes. Plant Cell. 1992;4:689–700. doi: 10.1105/tpc.4.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta-Gopalan C, Reichert NA, Barker RF, Hall TC, Kemp JD. Developmentally regulated epression of the beta-phaseolin gene in tobacco seed. Proc Natl Acad Sci U S A. 1985;82:3320–3324. doi: 10.1073/pnas.82.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewry PR, Halford NG. Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot. 2002;53(370):947–958. doi: 10.1093/jexbot/53.370.947. [DOI] [PubMed] [Google Scholar]
- Shewry PR, Tatham AS. Disulphide bonds in wheat gluten proteins. J Cereal Sci. 1997;25:207–227. doi: 10.1006/jcrs.1996.0100. [DOI] [Google Scholar]
- Shotwell MA, Larkins BA. The biochemistry and molecular biology of seed storage proteins. In: Marcus A, editor. The biochemistry of plants, a comprehensive treatise. New York: Academic; 1989. pp. 296–345. [Google Scholar]
- Shotwell MA, Boyer SK, Chesnut RS, Larkins BA. Analysis of seed storage protein genes of oats. J Biol Chem. 1990;265:9652–9658. [PubMed] [Google Scholar]
- Stoger E, Ma JKC, Fischer R, Christou P. Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotechnol. 2005;16:167–173. doi: 10.1016/j.copbio.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Takagi H, Hiroi T, Yang L, Takamura K, Ishimitsu R, Kawauchi H, Takaiwa F. Efficient induction of oral tolerance by fusing cholera toxin B subunit with allergen-specific T-cell epitopes accumulated in rice seed. Vaccine. 2008;26(48):6027–6030. doi: 10.1016/j.vaccine.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Ueda T, Waverczak W, Ward K. Mutations of the 22- and 27-kDa zein promoters affect transactivation by the Opaque-2 protein. Plant Cell. 1992;4:701–709. doi: 10.1105/tpc.4.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelosa M, Dattaa K, Olivaa N, Khalekuzzamana M, Torrizoa L, Krishnana S, Oliveirac M, Gotod F, Datta SK. Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci. 2003;164:371–378. doi: 10.1016/S0168-9452(02)00421-1. [DOI] [Google Scholar]
- Vicente-Carbajosa J, Moose SP, Parson RL, Schmidt RJ. A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque-2. Proc Natl Acad Sci U S A. 1997;94:7685–7690. doi: 10.1073/pnas.94.14.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JD, Galili G, Larkins BA, Gelvin SB. The synthesis of a 19 kilodalton zein protein in transgenic petunia plants. Plant Physiol. 1988;88:1002–1007. doi: 10.1104/pp.88.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Adachi T, Hatano T, Washida H, Suzuki A, Takaiwa F. Promoters of rice seed storage protein genes direct endosperm-specific gene expression in transgenic rice. Plant Cell Physiol. 1998;39(8):885–889. doi: 10.1093/oxfordjournals.pcp.a029449. [DOI] [Google Scholar]