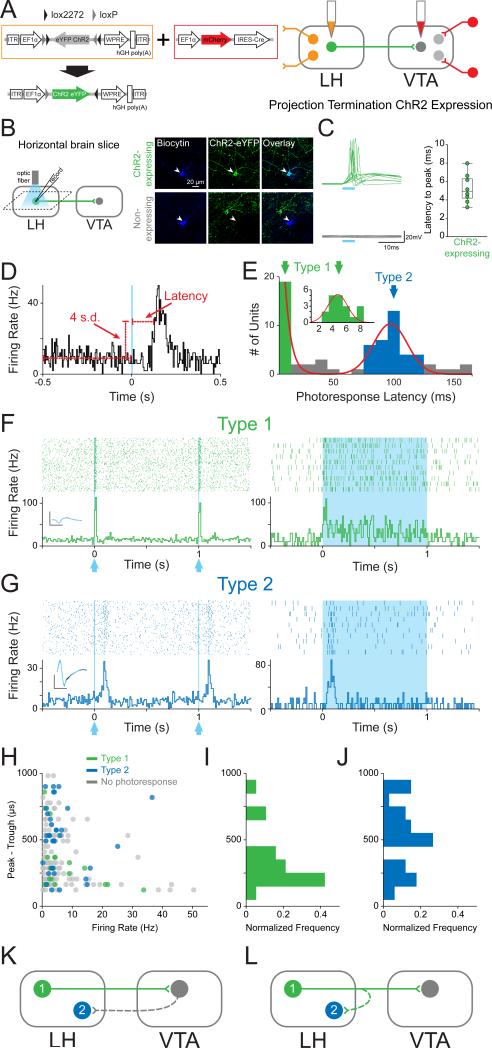
Figure 1. Phototagging LH-VTA projections reveals two populations of neurons with different response latencies to photostimulation.
(A) Wild-type mice (n=12) were injected with AAV5-DIO-ChR2-eYFP into the lateral hypothalamus (LH) and HSV-EF1α-IRES-Cre-mCherry into the ventral tegmental area (VTA). (B) Horizontal brain slices containing the LH were prepared for whole-cell patch-clamp recordings in ChR2-expressing and non-expressing LH neurons. (C) Individual traces recorded in current-clamp mode showing the response of ChR2-expressing (green, n=10) and non-expressing (grey, n=14) cells to a 5 ms pulse of 473 nm light. The box and whisker plot shows the average response latency for each ChR2-expressing cell ex vivo. (D) Photoresponse latencies in vivo were calculated by measuring the time from stimulation to 4 standard deviations (SD) above the baseline firing rate. (E) A bimodal distribution of excitatory photoresponse latencies was identified in recorded units (n=198) and divided into Type 1 (green; n=19) and Type 2 units (blue; n=34). (F) Type 1 units responded to photostimulation with fast excitation (3-8 ms latency). Inset shows the overlaid average traces for spontaneous spiking (black) and light-evoked spiking (blue) from a representative unit. (G) Type 2 units responded to photostimulation with delayed excitation (80-120 ms latency). (H) Scatterplot depicting the peak-trough duration of the waveform plotted against the average firing rate for each unit. (I) Normalized histogram showing the distribution of peak-trough durations for Type 1 units and (J) Type 2 units. (K and L) Diagrams illustrating two possible circuit models. (K) Type 1 units project directly from the LH to the VTA, while Type 2 units represent a population in the LH that is receiving feedback from the VTA, or (L) Type 2 units represent a population in the LH that is receiving input from collaterals of Type 1 units. Dotted lines indicate the presence of either a monosynaptic or polysynaptic connection. Scale bar: y-axis, 0.2 mV; x-axis, 500 μs. See also Figure S1.