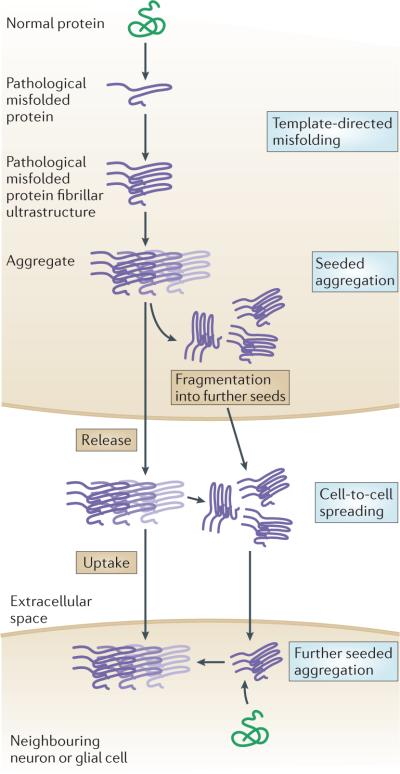
Figure 1. Hypothetical molecular mechanisms of prion-like disease protein transmission in neurodegenerative diseases.
In template-directed misfolding, the deposited pathological disease proteins are transformed from their normal conformation, via intermediates, into fibrillar species. These species have the properties of amyloid (for instance, a fibrillar ultrastructure that consists of sheets of β-strands) and serve as templates to drive normal physiological versions of the protein to adopt similar structural alterations13. In a self-perpetuating process, the progressive seeded aggregation of conformationally changed proteins results in intracellular aggregates that fragment into ‘daughter seeds’. Finally, in cell–cell transmission, pathological proteins spread to anatomically interconnected neurons and adjacent glial cells via an autocatalytic chain-reaction-like process11.