Fig 1.
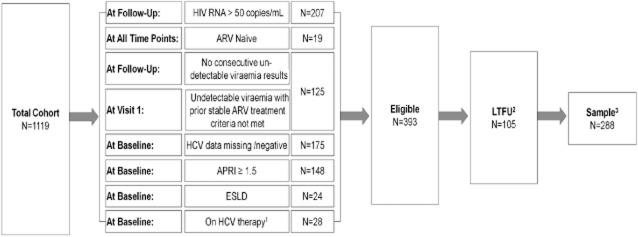
Participant selection flow chart. 1Hepatitis C virus (HCV) treatment censoring was carried out during the follow-up period. Antiretroviral (ARV) interruption was not censored. 2Participants not attending visits beyond study enrolment. 3The analysed data set is comprised of ‘valid risk-sets’. If one or multiple visits were skipped then that risk-set was excluded from analysis. APRI, aspartate aminotransferase to platelet ratio index; ESLD, end-stage liver disease; LTFU, lost to follow-up.
