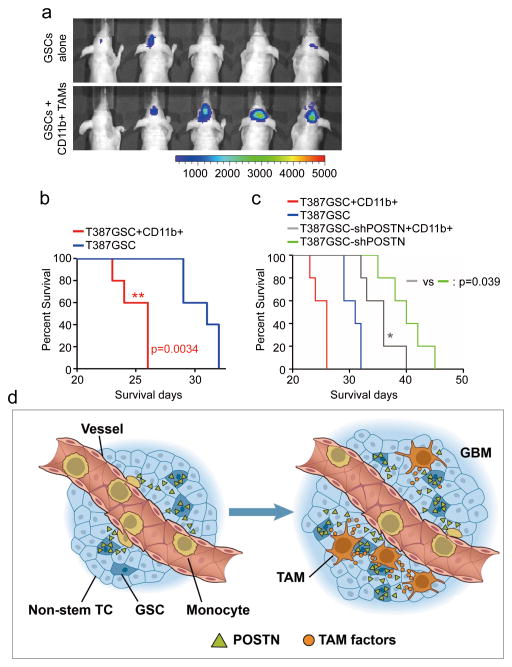
Figure 8. M2 subtype TAMs accelerated GSC tumor growth in mouse brains.
a, In vivo bioluminescent imaging analysis of tumor growth in mice bearing GBM xenografts derived from GSCs plus CD11b+ cells (enriched with M2 TAMs) or GSCs alone. 50, 000 CD11b+ cells were isolated from GSC-derived xenografts and then co-transplanted with 20, 000 GSCs intracranially into athymic nude mice. Representative images on day 18 post transplantation were shown (data from 5 mice). Co-transplantation of GSCs with CD11b+ TAMs dramatically promoted GSC tumor growth. b, Kaplan-Meier survival curves of mice implanted with GSCs plus CD11b+ cells or GSCs alone. Mice implanted with T387 GSCs plus CD11b+ cells showed a significantly shortened survival relative to the mice implanted with GSCs alone. p=0.0034. (n=5 mice for each group; two-tailed log-rank test). c, Kaplan-Meier survival curves of mice implanted with shPOSTN-expressing GSCs plus CD11b+ cells or shPOSTN-expressing GSCs alone. Mice implanted with shPOSTN-expressing GSCs (GSC-shPOSTN) plus CD11b+ cells showed a significantly reduced survival relative to the mice implanted with shPOSTN-expressing GSCs alone. p=0.039 (n=5 mice for each group; two-tailed log-rank test). d, A schematic representation of the POSTN-mediated recruitment of monocyte-derived TAMs from peripheral blood during GBM development. POSTN preferentially secreted by GSCs attracts monocytes from peripheral blood to enter GBM tissues. The POSTN-recruited, monocyte-derived TAMs are co-localized in perivascular niches with GSCs and maintained as M2 subtype macrophages that secret tumor supportive factors to promote GBM growth and progression.