Abstract
Objectives
Quaternary amine charge density is important because when the negatively-charged bacteria contact the positive quaternary amine charge, the electric balance is disturbed and the bacterium could be disrupted. There has been no report on the effects of charge density on the antibacterial efficacy of dental bonding agents. The objective of this study was to synthesize a new quaternary ammonium methacrylate, and investigate the effects of charge density of bonding agent on bacteria early-attachment, biofilm colony-forming units (CFU) and dentin bond strength.
Methods
Dimethylaminododecyl methacrylate (DMAHDM) with an alkyl chain length of 16 was synthesized and mixed into Scotchbond Multi-Purpose adhesive and primer (SBMP) at mass fractions of 0%, 2.5%, 5%, 7.5%, and 10%. A microtensile dentin bond test was performed. The density of quaternary ammonium groups was measured using a fluorescein dye method. Streptococcus mutans (S. mutans) early-attachment was examined at 4 hours, and biofilm colony-forming units (CFU) were measured at 2 days.
Results
All groups had similar microtensile bonding strengths (mean ± sd; n = 40) of about 60 MPa (p > 0.1). Quaternary amine charge density of bonding agents monotonically increased with increasing DMAHDM mass fraction. Bacteria early-attachment coverage greatly decreased with increasing DMAHDM content in the resin. Biofilm CFU at 10% DMAHDM was reduced by almost 5 log, compared to SBMP control. Charge density of bonding agent was inversely proportional to bacteria early-attachment coverage and biofilm CFU.
Significance
Increasing the quaternary amine charge density of dentin bonding agent resin was shown to greatly reduce S. mutans attachment and decrease biofilm CFU by four orders of magnitude, without compromising the dentin bond strength. The new DMAHDM is promising for use in bonding agents and other antibacterial restorative materials to inhibit caries.
Keywords: Antibacterial bonding agent, charge density, Streptococcus mutans, dentin bond strength, quaternary ammonium methacrylate, caries inhibition
1. Introduction
Secondary (recurrent) caries at the tooth-restoration margins has been suggested in previous studies as one of the primary reasons for restoration failure [1–4]. The replacement of failed restorations accounts for 50% or more of all the restorations performed [5,6], costing tens of billions of dollars annually. For example, the annual cost for tooth cavity restorations was approximately $46 billion in 2005 in the United States [7]. Furthermore, the need for tooth restorations is increasing rapidly with an aging population, longer life expectancy, and increased tooth retention in seniors [8]. Oral biofilms produce organic acids and enzymes which can lead to caries [1,9]. In addition, while resin composites are the principal material for cavity restorations [4,10–16], resins not only have no antibacterial function, but also may even accumulate more biofilms/plaques in vivo than other restorative materials [17,18]. Therefore, there is a need to develop antibacterial dental resins to inhibit biofilms and caries.
In previous studies, antibacterial resins containing quaternary ammonium methacrylates (QAMs) were synthesized [19–23]. 12-methacryloyloxydodecyl-pyridinium bromide (MDPB) could be copolymerized and covalently bonded in resins, thus becoming immobilized and exerting a contact-killing capability against oral bacteria and biofilms [24,25]. Several other antibacterial materials were recently reported, including a methacryloxylethyl cetyl dimethyl ammonium chloride (DMAE-CB)-containing adhesive [26], antibacterial glass ionomer cements [27], and antibacterial nanocomposites and bonding agents using a quaternary ammonium dimethacrylate (QADM) [28–30].
Bonding agents are used to adhere the restorations to tooth structures [31,32]. Extensive studies have improved the tooth-restoration bond strength and the understanding of the nature of adhesion [33–38]. Rendering the bonding agent antibacterial is meritorious in order to combat biofilm acids and recurrent caries at the tooth-restoration margins [24–26,39]. Besides residual bacteria in the prepared tooth cavity, marginal leakage would allow new bacteria to invade the tooth-restoration interface. Antibacterial bonding agents could help inhibit the residual as well as the invading bacteria [19,24,25]. Efforts were made to make both the primer and the adhesive resin to be antibacterial [19,26,29,30,40,41]. Regarding the antibacterial mechanism, quaternary ammonium salts (QAS) can cause bacteria lysis by binding to cell membrane to cause cytoplasmic leakage [39,42]. When the negatively charged bacteria contact the positive quaternary amine charge (N+), the electric balance is disturbed and the bacterium could explode under its own osmotic pressure [39,42]. Hence, the quaternary amine charge density is an important factor in the antibacterial efficacy. In a previous study, the charge density of poly(4-vinyl-N-alkylpyridinium bromide) coated glass slide was calculated as an index of surface properties [43]. Another study showed that the charge density of a dental resin increased with increasing the quaternary ammonium dimethacrylate mass fraction [21]. Although charge density evaluation was included in these reports, their main purpose was not to correlate these findings specifically with the efficiency of the antimicrobial agent. However, to date, the effects of quaternary amine charge density on the antibacterial efficacy and dentin bond strength of dental bonding agents have not been reported.
The objectives of this study were to: (1) synthesize a new QAM for incorporation into a dental bonding agent; and (2) systematically investigate the effects of quaternary amine charge density of bonding agent on bacteria early-attachment, biofilm colony-forming units (CFU) and dentin bond strength. The new QAM was incorporated into a primer and an adhesive at a series of mass fractions, thus allowing the quaternary amine charge density on the cured resin surface to be systematically varied. It was hypothesized that: (1) Increasing the charge density on bonding agent will monotonically decrease bacteria early-attachment; (2) Increasing the charge density of bonding agent resin will reduce the biofilm CFU; (3) Adding the QAM into bonding agent will not compromise the dentin bond strength, compared to the control without QAM.
2. Materials and methods
2.1. Development of new QAM and antibacterial bonding agent
Dimethylaminododecyl methacrylate (DMAHDM) with an alkyl chain length of 16 was synthesized using a modified Menschutkin reaction [21,28], where a tertiary amine group was reacted with an organo-halide. A benefit of this reaction is that the reaction products are generated at virtually quantitative amounts and require minimal purification [21]. Briefly, 10 mmol of 1-(dimethylamino)docecane (Sigma, St. Louis, MO) and 10 mmol of 1-bromohexadecane (BHD, TCI America, Portland, OR) were combined with 3 g of ethanol in a 20 mL scintillation vial. The vial was stirred at 70 °C for 24 h. The solvent was then removed via evaporation, yielding DMAHDM as a clear, colorless, and viscous liquid. Details of this method have been described recently [21,28,44].
Scotchbond Multi-Purpose adhesive and primer (referred as “SBMP”) (3M, St. Paul, MN) were used as the parent bonding system to test the effect of incorporation of antibacterial agent. According to the manufacturer, SBMP adhesive contained 60–70% of bisphenol A diglycidyl methacrylate (BisGMA) and 30–40% of 2-hydroxyethyl methacrylate (HEMA), tertiary amines and photo-initiator. SBMP primer contained 35–45% of HEMA, 10–20% of a copolymer of acrylic and itaconic acids, and 40–50% water. DMAHDM was mixed with SBMP primer at DMAHDM/(SBMP primer + DMAHDM) mass fraction of 2.5%, 5%, 7.5%, and 10%. Similarly, DMAHDM was mixed with SBMP adhesive at DMAHDM/(SBMP adhesive + DMAHDM) mass fraction of 2.5%, 5%, 7.5%, and 10%. The 10% mass fraction followed previous studies [29,30]. Therefore, five groups were tested:
Unmodified SBMP primer and adhesive (referred to as “SBMP control”);
SBMP primer + 2.5% DMAHDM, SBMP adhesive + 2.5% DMAHDM (“SBMP + 2.5% DMAHDM”);
SBMP primer + 5% DMAHDM, SBMP adhesive + 5% DMAHDM (“SBMP + 5% DMAHDM”);
SBMP primer + 7.5% DMAHDM, SBMP adhesive + 7.5% DMAHDM (“SBMP + 7.5% DMAHDM”);
SBMP primer + 10% DMAHDM, SBMP adhesive + 10% DMAHDM (“SBMP + 10% DMAHDM”).
2.2. Microtensile dentin bond strength
Human third molars were collected under a protocol approved by the University of Maryland Baltimore. The roots of teeth were removed via a water-cooled cutting saw (Isomet, Buehler, Lake Bluff, IL) [29,30]. The occlusal one-third of the tooth crown was removed to expose the mid-coronal dentin. The dentin surface was polished with 600-grit SiC paper, etched with 37% phosphoric acid gel for 15 s, and rinsed with distilled water. A primer was applied and the solvent was removed with a stream of air for 5 s. Then the corresponding adhesive was applied and light-cured for 10 s (Optilux VCL 401, Demetron Kerr, Danbury, CT). A composite (TPH, Caulk/Dentsply, Milford, DE) was applied and light-cured for 60 s [29,30]. After storage in de-ionized water at 37 °C for 24 h, each bonded tooth was vertically sectioned into 0.9 × 0.9 mm composite-dentin beams [45]. Eight teeth were used for each bonding agent group (n = 8). Five beams were randomly selected from the beams of each tooth, yielding 40 beams for each group. Each beam was stressed to failure in uniaxial tension using a computer-controlled Universal Testing Machine (MTS, Eden Prairie, MN) at a cross-head speed of 1 mm/min. The load-at-failure divided by the cross-sectional area at the site of failure yielded the microtensile dentin bond strength [45].
2.3. Quaternary amine charge density of bonding agent containing DMAHDM
The cover of a sterile 96-well plate (Costar, Corning Inc., Corning, NY) was used for fabricating resin disk specimens [26]. Ten µL of a primer was placed in the bottom of each dent. After drying with a stream of air, 20 µL of adhesive was applied to the dent and photo-polymerized for 10 s (Optilux VCL 401) using a Mylar strip covering to obtain a specimen disk of 8 mm in diameter and 0.5 mm in thickness. The cured disks were immersed in water and agitated for 1 h to remove uncured monomers, following a previous study [19]. The density of quaternary ammonium groups present on the polymer surfaces was quantified using a fluorescein dye method [21,43]. Resin disks of each bonding agent group were placed in a 48-well plate. Fluorescein sodium salt (200 µL of 10 mg/mL in deionized (DI) water) was added into each well, and specimens were left for 10 min at room temperature in the dark. After removing the fluorescein solution and rinsing extensively with DI water, each sample was placed in a new well, and 200 µL of 0.1% (by mass) of cetyltrimethylammonium chloride (CTMAC) in DI water was added. Samples were shaken for 20 min at room temperature in the dark to desorb the bound dye. The CTMAC solution was supplemented with 10% (by volume) of 100 mM phosphate buffer at pH 8. This was prepared with 0.94 mg/mL monosodium phosphate-monohydrate and 13.2 mg/mL disodium phosphate-anhydrous in DI water. Sample absorbance was read at 501 nm using a plate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA) [21,43]. The fluorescein concentration was calculated using Beers Law and an extinction coefficient of 77 mM−1 cm−1 [21,43]. Using a ratio of 1:1 for fluorescein molecules to the accessible quaternary ammonium groups, the surface charge density was calculated as the total molecules of charge per exposed surface area (the summation of top, bottom and side areas, measured independently for each polymer disk due to slight variations in disk sizes). Six replicates were tested for each group.
2.4. Bacteria early-attachment on bonding agent disks
Resin disk of 8 mm in diameter and 0.5 mm in thickness were sterilized with ethylene oxide (AnproleneAN 74i, Andersen, Haw River, NC) and de-gassed for 7 days. The use of Streptococcus mutans (S. mutans) (ATCC700610, American Type, Manassas, VA) was approved by the University of Maryland. S. mutans is a cariogenic bacterium and is the primary causative agent of caries. S. mutans was cultured overnight at 37 °C in Brain Heart Infusion (BHI, Becton, Sparks, MD) in an anaerobic atmosphere. The bacterial suspension was adjusted to an optical density (OD) of 0.06 at 600 nm for further usage. The overnight cultured bacterial suspension was diluted in fresh BHI until the OD value equaled to 0.06. Based on preliminary study on the relationship between OD value and CFU, the density of OD 0.06 was equivalent to a density of 107 S. mutans. Resin disks were placed in a 24-well plate with 1.5 mL of bacteria suspension, and incubated at 37 °C and 5% CO2 for 4 h, which was suitable for examining bacterial early attachment [21,46]. Disks were washed three times to remove nonadherent bacteria, fixed with 37 mg/mL formaldehyde, and stained for 1 h with 1 mol/L SYTOX green (Invitrogen, Carlsbad, CA) [46]. The stained disks were examined with an epifluorescence microscope (TE2000-S, Nikon, Melville, NY). Three disks were evaluated for each group. Five images were collected at random locations on each disk, yielding 15 images per group. Image J software (NIH) was used to measure the area coverage by bacteria in each image. The area coverage was normalized as a percentage of the SBMP control.
2.5. Anti-biofilm effect of bonding agent containing DMAHDM
Resin disks were placed in a 24-well plate with 1.5 mL of BHI supplemented with 0.2% sucrose as the culture medium. S. mutans bacterial suspension was diluted by 1:100, and then 10 µL of the suspension was inoculated into each well [26]. After incubating in 5% CO2 at 37 °C for 8 h, the disks were transferred to new 24-well plates with fresh medium. After 16 h, the disks were transferred to new 24-well plates with fresh medium and incubated for another 24 h. This constituted a total of 2 days of culture, which was shown previously to form mature biofilms on resins [29,30].
Disks with the 2-day biofilms were transferred into tubes with 2 mL of cysteine peptone water (CPW), and the biofilms were harvested by sonication (3510R-MTH, Branson, Danbury, CT) for 3 min, and then vortexing at maximum speed for 20 s using a vortex mixer (Fisher, Pittsburgh, PA). The bacterial suspensions thus obtained were serially diluted, spread onto agar plates for CFUs analysis [29,30]. Six replicates were tested for each group.
2.6. Statistical analysis
All data were checked for normal distribution with the Kolmogorov-Smirnov test and were checked for homogeneity with the Levene’s test. Inter-group differences were estimated by a statistical analysis of variance (ANOVA) for factorial models; individual groups were compared with Fisher’s protected least-significant difference test. Statistical analyses were performed by SPSS 13.0 software (SPSS, Chicago, IL) at a pre-set alpha of 0.05.
3. Results
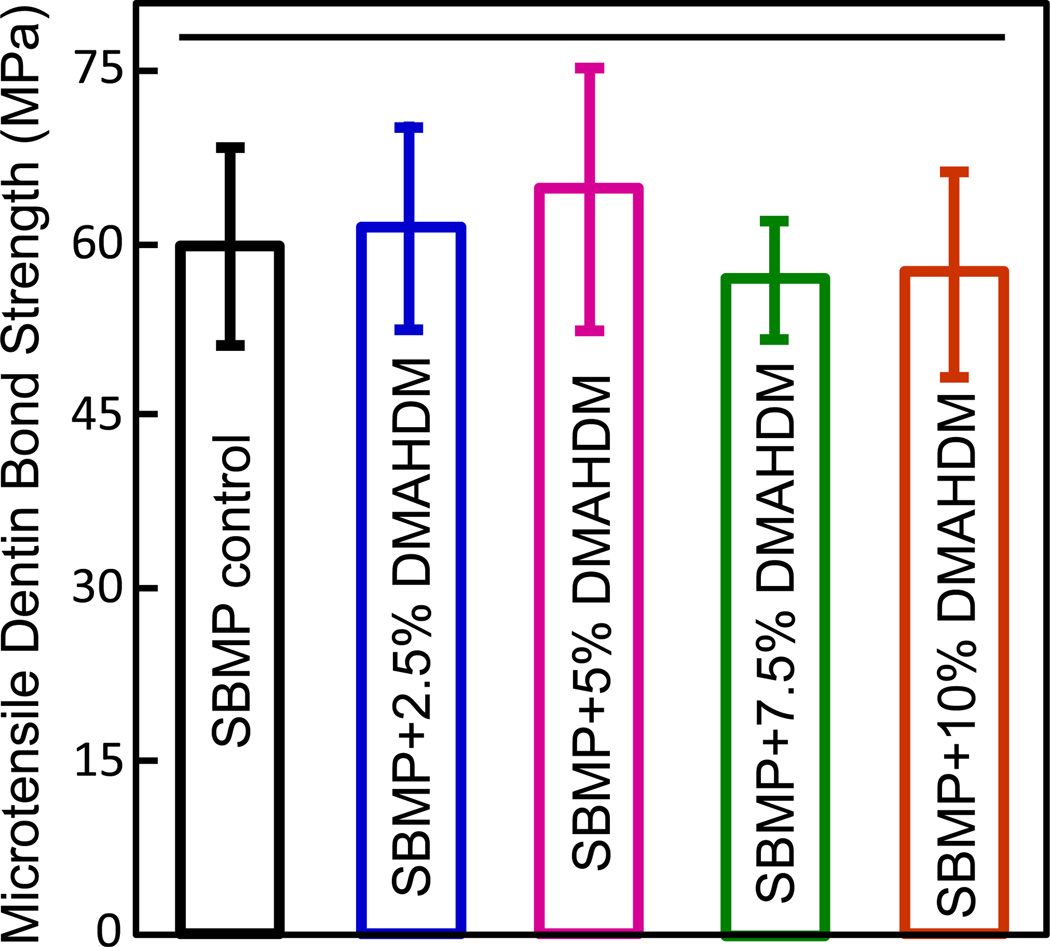
The microtensile dentin bond strengths are plotted in Fig. 1 (mean ± sd; n = 8). All groups had bond strengths of about 60 MPa, with no statistically significant difference among them (p > 0.1). Therefore, the incorporation of DMAHDM into SBMP bonding agent at the tested concentrations did not compromise the dentin bonding strength.
Fig. 1.
Human dentin microtensile bond strength for the five groups of bonding agents (mean ± sd; n = 8). Horizontal line indicates that there was no significant difference between the groups (p > 0.1).
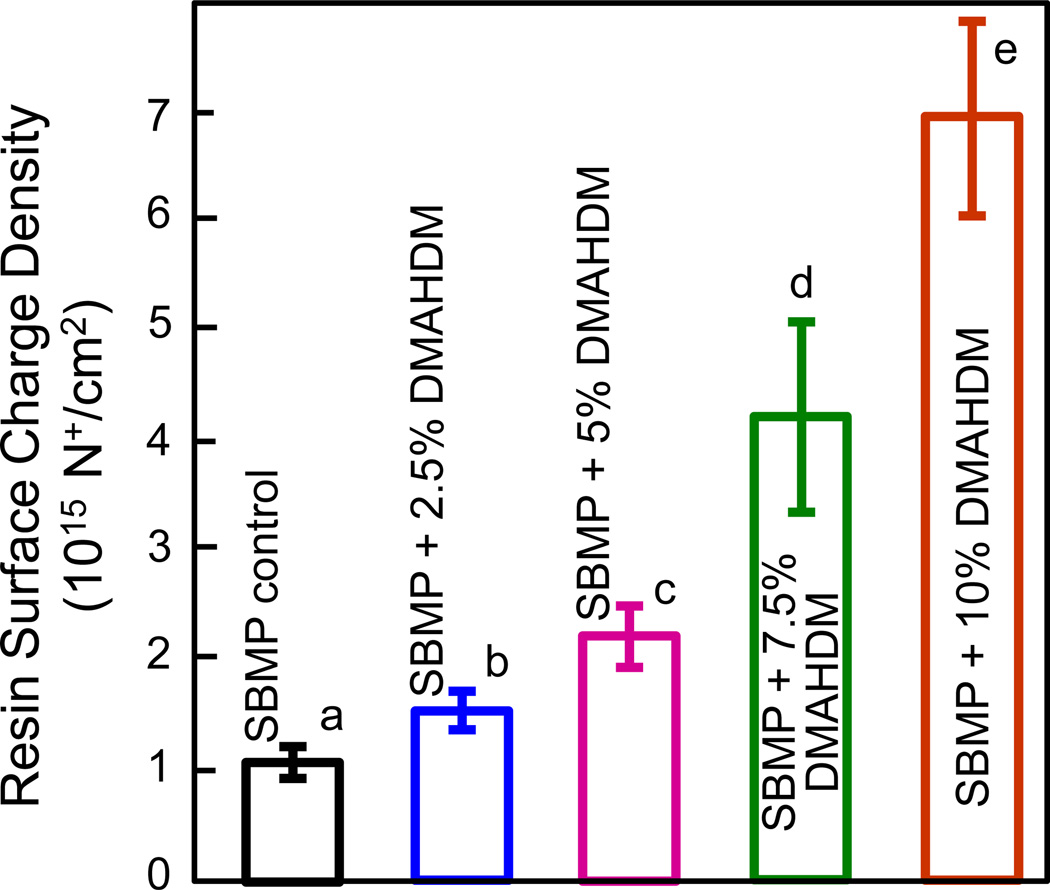
The quaternary amine surface charge density of bonding agents containing DMAHDM is plotted in Fig. 2 (mean ± sd; n = 6). Fluorescein binding to the cationic quaternary groups revealed statistically significant increases in the quaternary ammonium sites present on the surfaces of the cured bonding agent with increasing the DMAHDM concentration (p < 0.05). Resin disks with 10% DMAHDM had approximately 5 times more quaternary ammonium sites present on the surfaces as compared with the 2.5% group. Samples with 0% DMAHDM had slight nonspecific interaction with and absorption of the fluorescein salt.
Fig. 2.
Quaternary amine surface charge density of the cured bonding agent resin disks vs. DMAHDM mass fraction (mean ± sd; n = 6). Surface charge density significantly increased with increasing the DMAHDM mass fraction; values with dissimilar letters are significantly different from each other (p < 0.05).
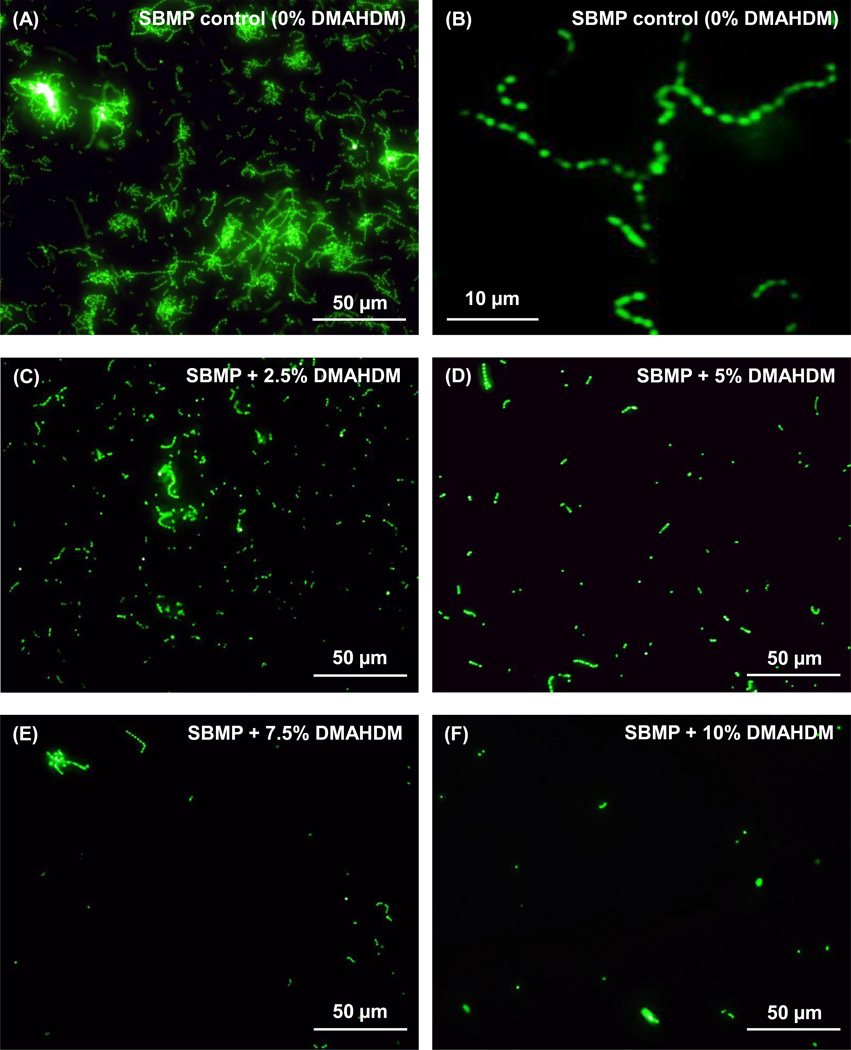
Fig. 3 shows representative fluorescent images of S. mutans early-attachment and contact-killing effects of bonding agents containing DMAHDM: (A) SBMP control, (C) SBMP + 2.5% DMAHDM, (D) SBMP + 5% DMAHDM, (E) SBMP + 7.5% DMAHDM, (F) SBMP + 10% DMAHDM. A higher magnification for SBMP control with 0% DMAHDM is shown in (B). S. mutans were incubated on resin disks for 4 h. Incorporation of DMAHDM reduced bacteria colonization for all tested concentrations, and the colonization decreased with increasing the DMAHDM content. In addition, the bacterial morphology was altered on polymers containing 7.5% or 10% DMAHDM, compared to SBMP control. S. mutans long chains were frequently observed in (A) and more clearly shown in (B) on the control. The chains were much shorter in (C–E). At 10% DMAHDM, only individual bacteria dots were observed (F).
Fig. 3.
Typical fluorescent images of S. mutans colonization on bonding agent resin disks containing DMAHDM: (A) SBMP control, (C) SBMP + 2.5% DMAHDM, (D) SBMP + 5% DMAHDM, (E) SBMP + 7.5% DMAHDM, (F) SBMP + 10% DMAHDM. A higher magnification for SBMP control is shown in (B). S. mutans were incubated on resin disks for 4 h. S. mutans had long chains in (A) which is more clearly shown in (B) on SBMP control, versus individual bacteria dots at 10% DMAHDM in (F).
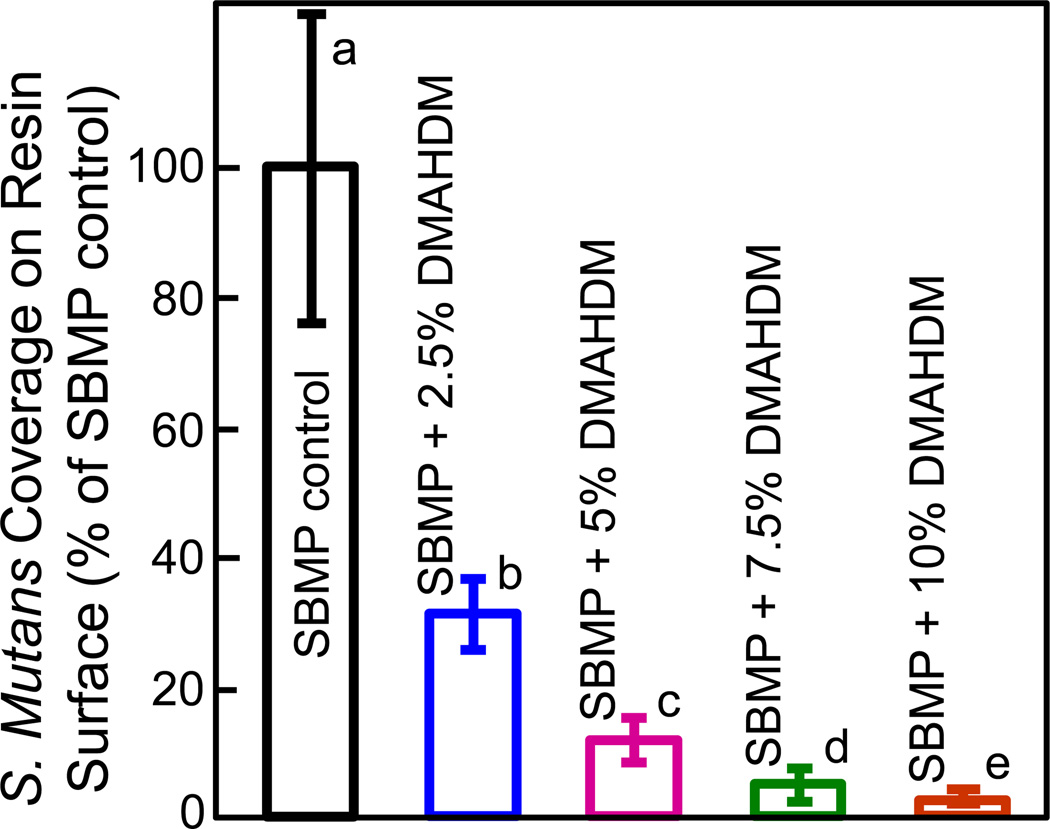
The percentage of surface area covered by bacteria is plotted in Fig. 4 (mean ± sd; n = 15). S. mutans were cultured on resin disks for 4 h to examine early-attachment and contact-killing effects. The S. mutans coverage area monotonically decreased with increasing the DMAHDM mass fraction in the bonding agent. All groups were significantly different from each other (p < 0.05).
Fig. 4.
S. mutans coverage on cured bonding agent resin disks vs. DMAHDM mass fraction (mean ± sd; n = 15). S. mutans were cultured on resin disks for 4 h to examine early-attachment. The S. mutans surface area coverage on resin with DMAHDM/S. mutans surface area on SBMP control yielded the percentage of bacteria coverage. S. mutans coverage area greatly decreased with increasing the DMAHDM mass fraction. Values with dissimilar letters are significantly different from each other (p < 0.05).
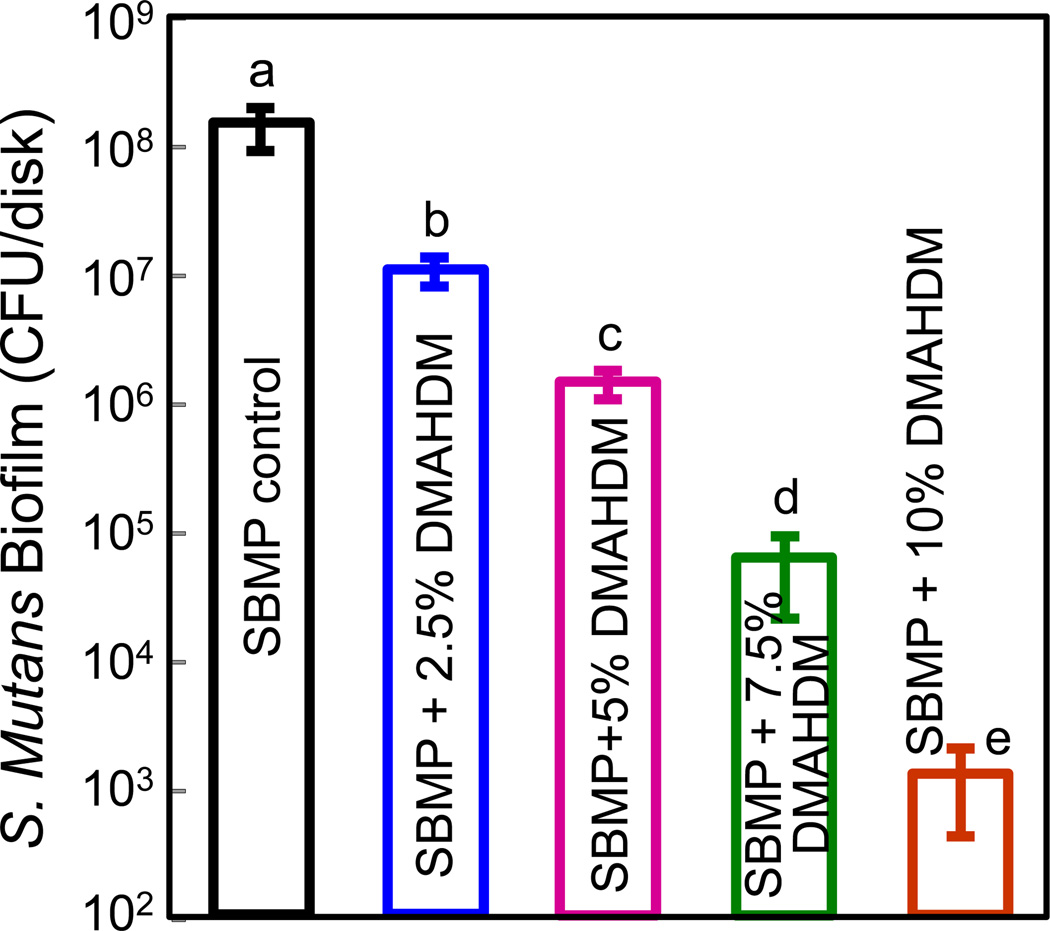
The anti-biofilm effect of DMAHDM-incorporating bonding systems is shown in Fig. 5 (mean ± sd; n = 6). S. mutans biofilms were grown on the cured bonding agent resin surfaces for 2 days. The SBMP control group yielded the highest CFU value. With increasing DMAHDM concentration, the bacterial quantity in biofilm decreased (p < 0.05). Note that the y-axis is a log scale. Bacterial amount for the 10% DMAHDM group was reduced by almost 5 orders of magnitude compared to SBMP control, indicating the strongest anti-biofilm effect among all the tested groups. All the groups were significantly different from each other (p < 0.05).
Fig. 5.
S. mutans biofilm CFU vs. DMAHDM mass fraction in bonding agent (mean ± sd; n = 6). Biofilms were cultured on the cured bonding agent resin disks for 2 days. The y-axis is a log scale. Biofilm CFU for the 10% DMAHDM group was reduced by almost 5 orders of magnitude compared to SBMP control. Values with dissimilar letters are significantly different from each other (p < 0.05).
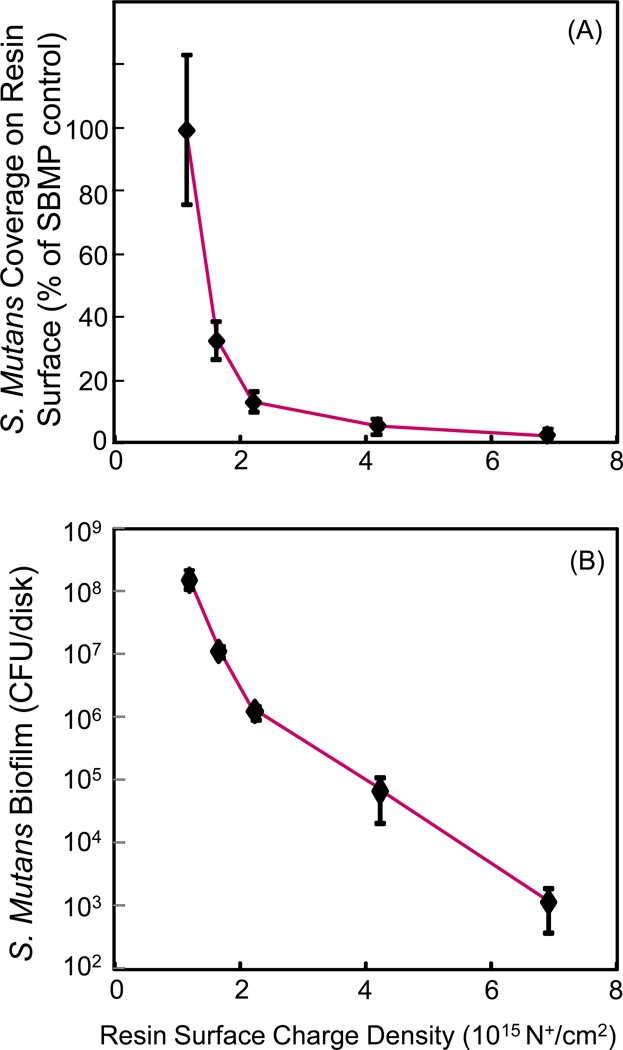
The effect of surface charge density is shown in Fig. 6 versus bacteria early-attachment at 4 hours (A), and 2-day biofilm CFU (B). The charge density of bonding agent resins was inversely proportional to bacteria early-attachment and biofilm growth. Both the bacteria earlyattachment and biofilm CFU values were greatly reduced when the surface charge density was increased with increasing the DMAHDM mass fraction in the resin, reaching 7×1015 N+/cm2 at 10% DMAHDM.
Fig. 6.
Antibacterial efficacy of bonding agent vs. quaternary amine surface charge density: (A) Bacteria early-attachment at 4 hours, (B) 2-day biofilm CFU (mean ± sd; n = 6). Bonding agent charge density was strongly inversely-proportional to bacteria early-attachment and biofilm CFU. The binding agent contained DMAHDM at different mass fractions to obtain a series of charge densities.
4. Discussion
The hypotheses were supported by the data of the present study. Increasing the charge density of dental bonding agent greatly reduced the bacterial early-attachment, and decreased the biofilm CFU by four orders of magnitude. These antibacterial functions were obtained without decreasing the dentin microtensile bond strength, compared to the commercial parent bonding agent without QAM. Considering that recurrent caries at the tooth-restoration margins is a primary reason for restoration failure which costs tens of billions of dollars annually, the antibacterial bonding agent containing the new DMAHDM at a relatively high charge density would be beneficial. The positively-charged quaternary amine N+ of a QAM can attract the negatively-charged cell membrane of bacteria, which can disrupt the cell membrane and cause cytoplasmic leakage [39,42]. In addition, the coupling of the positively-charged ammonium group with the negatively-charged bacterial membrane can alter the balance of essential ions (i.e., K+, Na+, Ca2+, and Mg2+), interrupt protein activity, and damage bacterial DNA [47]. Indeed, a previous study prepared antimicrobial polymeric brushes and showed that high density cationic surfaces effectively killed bacterial cells [48]. Another study showed that the charge density of a dental resin increased with increasing the quaternary ammonium dimethacrylate mass fraction [21]. However, there has been little study on the effects of charge density on antibacterial properties of dental resins. A literature search revealed that the present study represents the first report on systematically varying the quaternary amine charge density of a dental bonding agent, and determining its effect on bacteria early-attachment, biofilm growth, and dentin bond strength.
The mechanism of positively-charged quaternary amine disrupting the negatively-charged bacterial membranes could explain the results of the present study, showing that a resin with a higher density of positive charges had a higher antibacterial potency. The bacterial morphology was altered on resin surfaces with 5% to 10% DMAHDM compared to control. The S. mutans changed from long chains to short chains and individual cells, and the cell shape changed from round bacteria to deformed ones indicating damage to bacterial membranes. For early attachment at 4 h, the S. mutans coverage decreased rapidly with increasing charge density, losing nearly 90% of bacterial coverage at 5% DMAHDM, compared to that of SBMP control. The rate of decrease in bacterial coverage then slowed from 5% to 10% DMAHDM. A similar trend was observed in 2-day S. mutans biofilm CFU, in which the slope of CFU decrease was steeper from 0% to 5% DMAHDM, than that from 5% to 10% DMAHDM. Further study is needed to understand why the bacterial coverage and CFU are not linearly proportional to the charge density. One possibility is that at a lower DMAHDM concentration, there were less positive charges on the resin surface, and all the positive charges were interacting with bacteria to exert antibacterial effects. In comparison, at a high DMAHDM concentration, the charge density may exceed the amount of bacteria, hence only a portion of the positive charges were interacting with bacteria to exert an antibacterial activity. Hence the antibacterial activity was not linearly proportional to the charge density.
Caries is a dietary carbohydrate-modified bacterial infectious disease caused by the secretion of acids from biofilms. When a tooth decay is removed, it is usually impossible to completely remove all the bacteria present. There are often residual bacteria left in the prepared tooth cavity, sometimes due to the purpose of preserving more tooth structure or avoiding pulp perforation [20,24]. This is especially true with the increased interest in less removal of tooth structure and in the practice of minimal intervention dentistry [49,50], in which more carious tissues with active bacteria are expected to be left in the tooth cavity. The antibacterial primer of the present study containing DMAHDM would be useful in killing the residual bacteria in the prepared tooth cavity because the primer has direct contact with the tooth cavity and flows into dentinal tubules. In addition, the adhesive of the present study containing DMAHDM would also be beneficial. This is because besides residual bacteria in the tooth cavity, there are new invading bacteria at the tooth-restoration interfaces in vivo. While a perfect seal at the margins is highly desirable, it is often difficult to achieve. The tooth-restoration interface could degrade due to polymerization shrinkage stresses and cyclic fatigue and wear actions. Therefore, microgaps could be present at the tooth-restoration margins [51–53]. Biofilms could invade these microgaps which would be difficult to clean by tooth brushing, and the acid production could cause demineralization at the margin to further degrade the interface. Previous studies observed microgaps between the adhesive resin and the primed dentin, or between the adhesive resin and the hybrid layer [51,53]. This suggests that a large portion of the marginal gap would be surrounded by the cured adhesive resin, and the invading bacteria would come into contact with the adhesive surface [24]. These facts suggest that an antibacterial adhesive would be important to combat the invading bacteria at the margins. This was why both the primer and the adhesive contained DMAHDM in the present study. The new antibacterial primer and adhesive, especially those containing 10% DMAHDM with a relatively high charge density, could reduce biofilm CFU by 4 orders of magnitude, and therefore are highly promising to inhibit biofilm growth and recurrent caries at the margins.
Regarding the durability of the antibacterial function, previous studies showed that the incorporation of QAMs in resins yielded a long-term antibacterial activity, because the antibacterial agent was copolymerized with the resin by forming a covalent bonding with the polymer network [20,24]. Therefore, the antibacterial agent was immobilized in the resin matrix, and was not released or lost over time, thus providing a durable antibacterial capability [20,24]. Indeed, the antibacterial effect of MDPB composite was maintained after water-aging for 3 months [54], and an adhesive containing DMAE-CB showed no decrease in anti-biofilm effect after water-aging for 1 month [55]. A recent study showed that the antibacterial efficacy of a nanocomposite containing a QAM after water-aging for 6 months was not significantly different from that at 1 day [29]. Another study on bonding agents containing dimethylaminododecyl methacrylate demonstrated that the anti-biofilm properties had no significant decrease in water-aging from 1 day to 6 months [56]. In addition, that study showed that after 6 months of water-aging, the commercial bonding agent lost 1/3 of its dentin bond strength; in contrast, the new antibacterial bonding agent exhibited no loss of dentin bond strength in 6 months [56].
The present study has two main limitations. First, it is a short-term study without examining the long-term durability of the antibacterial function and dentin bond strength. Second, it is an in vitro study without complications of the oral environment such as saliva, food debris and wear in vivo. Further studies are needed to determine the antibacterial activity and dentin bond strength of bonding agents containing the new DMAHDM versus time, as well as the antibacterial efficacy in tooth restorations in an animal model.
5. Conclusions
A new antibacterial monomer dimethylaminododecyl methacrylate (DMAHDM) with an alkyl chain length of 16 was incorporated into bonding agent at different mass fractions, thus enabling the variation of the quaternary amine charge density on the resin. This in turn enabled the investigation of the effects of charge density of bonding agent on bacteria early-attachment, biofilm CFU, and dentin bond strength.
Increasing the charge density on bonding agent greatly reduced S. mutans early-attachment and decreased biofilm CFU by 4 log.
Antibacterial functions were obtained without negatively affecting dentin bond strength, compared to commercial control.
The novel antibacterial bonding agents are promising for killing residual bacteria in the tooth cavity and inhibiting bacteria at the margins. DMAHDM may be useful in other types of bonding agents, cements, sealants and composites to inhibit caries.
Acknowledgments
We thank Drs. Joseph M. Antonucci, Nancy J. Lin and Sheng Lin-Gibson of the National Institute of Standards and Technology, and Ashraf F. Fouad of the University of Maryland School of Dentistry for discussions. We thank Dr. Huaibing Liu at Caulk/Dentsply (Milford, DE) for donating the TPH composite. This study was supported by NIH R01 DE17974 (HX), National Natural Science Foundation of China grant 81100772 (FL), and a bridge funding from the Department of Endodontics, Prosthodontics and Operative Dentistry of the University of Maryland School of Dentistry (HX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mjor IA, Toffeneti F. Secondary caries: a literature review with caries reports. Quintessence International. 2000;31:165–179. [PubMed] [Google Scholar]
- 2.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dental Materials. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dental Materials. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Ferracane JL. Resin composite - State of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Primary Dental Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 6.National Institute of Dental and Craniofacial Research (NIDCR) announcement # 13-DE-102. Dental Resin Composites and Caries. 2009 Mar 5; [Google Scholar]
- 7.Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. Economic impact of regulating the use of amalgam restorations. Public Health Report. 2007;122:657–663. doi: 10.1177/003335490712200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saunders RH, Meyerowitz C. Dental caries in older adults. Dental Clinics of North America. 2005;49:293–308. doi: 10.1016/j.cden.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. Journal of Dental Research. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 10.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dental Materials. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 11.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dental Materials. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. Journal of Dental Research. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coelho-De-Souza FH, Camacho GB, Demarco FF, Powers JM. Fracture resistance and gap formation of MOD restorations: influence of restorative technique, bevel preparation and water storage. Operative Dentistry. 2008;33:37–43. doi: 10.2341/07-27. [DOI] [PubMed] [Google Scholar]
- 14.Samuel SP, Li S, Mukherjee I, Guo Y, Patel AC, Baran GR, et al. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dental Materials. 2009;25:296–301. doi: 10.1016/j.dental.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: Not only a matter of materials. Dental Materials. 2012;28:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Satterthwaite JD, Maisuria A, Vogel K, Watts DC. Effect of resin-composite filler particle size and shape on shrinkage-stress. Dental Materials. 2012;28:609–614. doi: 10.1016/j.dental.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. Accumulation of Streptococcus mutans on light-cured composites and amalgam: An in vitro study. Journal of Esthetic Dentistry. 1998;10:187–190. doi: 10.1111/j.1708-8240.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 18.Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. Journal of Dentistry. 2007;35:201–206. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. Journal of Dentistry. 1998;26:267–271. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 20.Imazato S. Bio-active restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dental Materials Journal. 2009;28:11–19. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 21.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–228. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weng Y, Howard L, Guo X, Chong VJ, Gregory RL, Xie D. A novel antibacterial resin composite for improved dental restoratives. Journal of Materials Science. 2012;23:1553–1561. doi: 10.1007/s10856-012-4629-z. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:1151–1162. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–319. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 25.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dental Materials. 2007;23:170–176. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. Journal of Dentistry. 2009;37:289–296. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dental Materials. 2011;27:487–496. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, Lin-Gibson S, Zhou X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dental Materials. 2012;28:561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Journal of Dental Research. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dental Materials. 2012;28:842–852. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer P, Ye Q, Park JG, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL. Adhesive/dentin interface: The weak link in the composite restoration. Annals of Biomedical Engineering. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. Journal of Biomedical Materials Research. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 34.Ikemura K, Tay FR, Endo T, Pashley DH. A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dental Materials Journal. 2008;27:315–329. doi: 10.4012/dmj.27.315. [DOI] [PubMed] [Google Scholar]
- 35.Ritter AV, Swift EJ, Jr, Heymann HO, Sturdevant JR, Wilder AD., Jr An eight-year clinical evaluation of filled and unfilled one-bottle dental adhesives. Journal of the American Dental Association. 2009;140:28–37. doi: 10.14219/jada.archive.2009.0015. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Godoy F, Kramer N, Feilzer AJ, Frankenberger R. Long-term degradation of enamel and dentin bonds: 6-year results in vitro vs. in vivo. Dental Materials. 2010;26:1113–1118. doi: 10.1016/j.dental.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Roeder L, Pereira RNR, Yamamoto T, Ilie N, Armstrong S, Ferracane J. Spotlight on bond strength testing-Unraveling the complexities. Dental Materials. 2011;27:1197–1203. doi: 10.1016/j.dental.2011.08.396. [DOI] [PubMed] [Google Scholar]
- 38.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J. State of the art of self-etch adhesives. Dental Materials. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 39.Namba N, Yoshida Y, Nagaoka N, Takashima S, Matsuura-Yoshimoto K, Maeda H, Van Meerbeek B, Suzuki K, Takashiba S. Antibacterial effect of bactericide immobilized in resin matrix. Dental Materials. 2009;25:424–430. doi: 10.1016/j.dental.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dental Materials. 2006;22:527–532. doi: 10.1016/j.dental.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Hiraishi N, Yiu CK, King NM, Tay FR. Effect of chlorhexidine incorporation into a selfetching primer on dentine bond strength of a luting cement. Journal of Dentistry. 2010;38:496–502. doi: 10.1016/j.jdent.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 42.Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Tiller J, Liao C, Lewis K, Klibanov A. Designing surfaces that kill bacteria on contact. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HH. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013 doi: 10.1016/j.jdent.2013.01.004. http://dx.doi.org/10.1016/j.jdent.2013.01.004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li F, Liu XY, Zhang L, Kang JJ, Chen JH. Ethanol-wet bonding technique may enhance the bonding performance of contemporary etch-and-rinse dental adhesives. Journal of Adhesive Dentistry. 2012;14:113–120. doi: 10.3290/j.jad.a21853. [DOI] [PubMed] [Google Scholar]
- 46.Zeiger DN, Stafford CM, Cheng Y, Leigh SD, Lin-Gibson S, Lin NJ. Effects of sample preparation on bacterial colonization of polymers. Langmuir. 2010;26:2659–2664. doi: 10.1021/la902920n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simoncic B, Tomcis B. Structures of novel antimicrobial agents for textiles - a review. Textile Research Journal. 2010;80:1721–1737. [Google Scholar]
- 48.Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. Permanent, non-leaching antibacterial surfaces -2: How high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28:4870–4879. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 49.Tyas MJ, Anusavice KJ, Frencken JE, Mount GJ. Minimal intervention dentistry - a review FDI commission project 1–97. International Journal of Dentistry. 2000;50:1–12. doi: 10.1111/j.1875-595x.2000.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 50.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. Journal of the American Dental Association. 2011;142:612–620. doi: 10.14219/jada.archive.2011.0243. [DOI] [PubMed] [Google Scholar]
- 51.Perdigao J, Lambrechts P, Van Meerbeek B, Braem M, Yildiz E, Yucel T, Vanherle G. The interaction of adhesive systems with human dentin. American Journal of Dentistry. 1996;9:167–173. [PubMed] [Google Scholar]
- 52.Fruits TJ, Knapp JA, Khajotia SS. Microleakage in the proximal walls of direct and indirect posterior resin slot restorations. Operative Dentistry. 2006;31:719–727. doi: 10.2341/05-148. [DOI] [PubMed] [Google Scholar]
- 53.Loguercio AD, Reis A, Bortoli G, Patzlaft R, Kenshima S, Rodrigues LE, Accorinte Mde L, van Dijken JW. Influence of adhesive systems on interfacial dentin gap formation in vitro. Operative Dentistry. 2006;31:431–441. doi: 10.2341/05-53. [DOI] [PubMed] [Google Scholar]
- 54.Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. Incorporation of bacterial inhibitor into resin composite. Journal of Dental Research. 1994;73:1437–1443. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- 55.Li F, Chai ZG, Sun MN, Wang F, Ma S, Zhang L, Fang M, Chen JH. Anti-biofilm effect of dental adhesive with cationic monomer. Journal of Dental Research. 2009;88:372–376. doi: 10.1177/0022034509334499. [DOI] [PubMed] [Google Scholar]
- 56.Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing antibacterial monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013 doi: 10.1016/j.jdent.2013.03.011. accepted for publication). [DOI] [PMC free article] [PubMed] [Google Scholar]