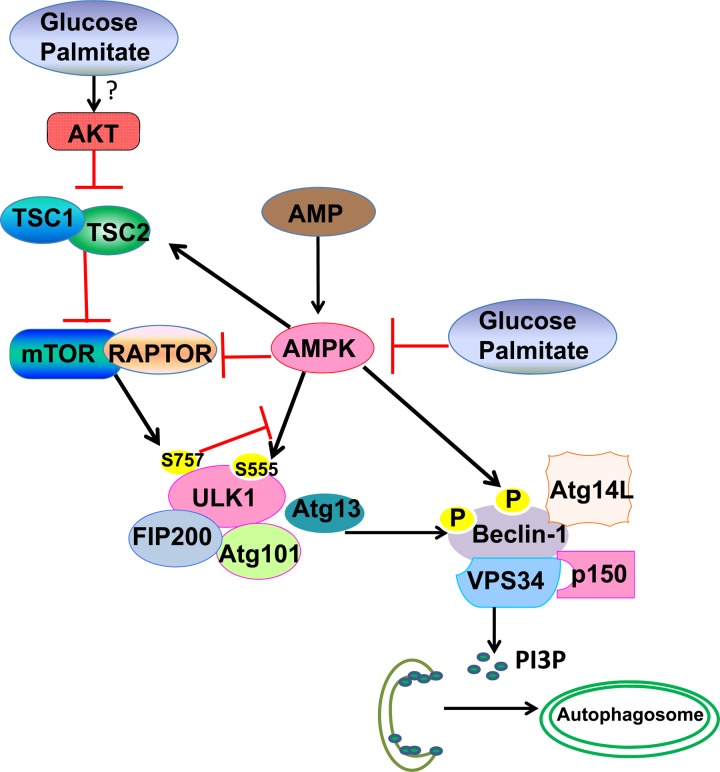
Fig. 1.
A proposed model for how glucose and palmitate impair the AMP-activated protein kinase (AMPK)-mammalian target of rapamycin (mTOR)-Unc-51-like kinase 1 (ULK1) signaling cascade in autophagy. High nutrients may activate mTOR through Akt activation (although this remains to be further determined in the future under glucose and palmitate conditions). Glucose and palmitate inactivate AMPK, which is a positive regulator for autophagy at three layers of regulation: suppression of mTOR activity through phosphorylation of TSC2 and RAPTOR; phosphorylation of ULK1 (S555); and promotion of VPS34 kinase activity through phosphorylation of Beclin 1. Activated VPS34 increases the production of phosphatidylinositol 3-phosphate (PI3P), which promotes the biogenesis of autophagosomes. mTOR negatively regulates autophagy through direct phosphorylation of ULK1 (S757) to inactivate ULK1 activity. ULK1 directly phosphorylates Beclin 1 and enhances VPS34 kinase activity to promote autophagy. mTOR-mediated phosphorylation of ULK1 (S757) also prevents ULK1 interaction with AMPK and thus suppresses the phosphorylation of ULK1 (S555) by AMPK to activate ULK1 and autophagy. mTOR-mediated blockage of the interaction of AMPK with ULK1 is most likely the mechanism for how AMPK was uncoupled from autophagy under glucose and palmitate conditions in human aortic endothelial cells (HAECs).