Abstract
Based on type studies and freshly collected material we here re-instate the genus Thyronectria (Nectriaceae, Hypocreales). Species of this genus were recently for the most part classified in the genera Pleonectria (Nectriaceae) or Mattirolia (Thyridiaceae), because Thyronectria and other genera had been identified as members of the Thyridiaceae due to the presence of paraphyses. Molecular phylogenies based on several markers (act, ITS, LSU rDNA, rpb1, rpb2, tef1, tub) revealed that the Nectriaceae contain members whose ascomata are characterised by long, more or less persistent, apical paraphyses. All of these belong to a single genus, Thyronectria, which thus has representatives with hyaline, rosy, green or even dark brown and sometimes distoseptate ascospores. The type species of Thyronectria, T. rhodochlora, syn. T. patavina, syn. T. pyrrhochlora is re-described and illustrated. Within the Nectriaceae persistent, apical paraphyses are common in Thyronectria and rarely also occur in Nectria. The genus Mattirolia is revised and merged with Thyronectria and also Thyronectroidea is regarded as a synonym of Thyronectria. The three new species T. asturiensis, T. caudata and T. obscura are added to the genus. Species recently described in Pleonectria as well as some species of Mattirolia are combined in the genus, and a key to Thyronectria is provided. Five species are epitypified. The type species of the genus Thyridium (Thyridiaceae), T. vestitum, is included in phylogenetic analyses to illustrate the phylogenetic distance of Thyronectria from the Thyridiaceae.
Keywords: act, Ascomycota, Hypocreales, Mattirolia, Nectriaceae, new species, Pleonectria, pyrenomycetes, rpb1, rpb2, tef1, tub, Thyridiaceae, Thyridium, Thyronectroidea
INTRODUCTION
The family Nectriaceae includes about 20 genera of which two major genera were monographed by Hirooka et al. (2012) in a comprehensive and voluminous work. One of the generic names revived in that work is Pleonectria Sacc. (1876). The genus Thyronectria Sacc. (1875), based on T. patavina Sacc., is older and has therefore priority over Pleonectria. As described by Saccardo (1875a: 21), the generic name refers to superficial similarities of the immersed stromata and muriform ascospores to the genus Thyridium, whereas the nectriaceous perithecial context and the hyaline ascospores being similar to Nectria or Calonectria. Seeler (1940) monographed this genus, which he characterised by light-coloured perithecia immersed in or superficial on erumpent stromata, often clothed with yellowish or greenish scales or powder, with eventually ‘evanescent pseudoparaphyses’ and hyaline, yellowish, green or brown, muriform ascospores that may form conidia by budding in the ascus. He determined the asexual morphs to belong to Gyrostroma, Dendrodochium and Stilbella. Hamathecial elements in the Hypocreales have been determined to be restricted to periphyses and apical paraphyses, while true paraphyses, i.e. sterile filaments emerging from the subhymenium between asci, are not known in the Hypocreales. Apical paraphyses develop in a cushion at the top of the perithecium and grow downward to the bottom of the perithecial cavity, forming a compact palisade or appearing like a pseudoparenchyma. After growth of asci into this palisade, the apical paraphyses become disintegrated and are not present between mature asci (Hanlin 1961, 1971). Zhang et al. (2006) used the absence of true paraphyses as a typical character of the subclass Hypocreomycetidae of the Sordariomycetes.
When Rossman et al. (1999) detected paraphyses in the holotype of T. patavina, they concluded that Thyronectria was not available for muriform-spored nectriaceous species, and they referred the genus Thyronectria to the Thyridiaceae. They placed other species regarded as Thyronectria by Seeler (1940) in the genus Nectria. Rossman et al. (1999) relegated also Balzania Speg., Mattirolia Berl. & Bres. and Thyronectroidea Seaver to the Thyridiaceae. In a morphotaxonomic work, Checa et al. (2013) accepted the placement of the latter genera in the Thyridiaceae and recognised Balzania and Thyronectroidea as synonyms of Mattirolia.
The species classified by Rossman et al. (1999) in Nectria were revised by Hirooka et al. (2012), who recognized three genera. The authors also determined that yellow scurf on ascomata or stromata is confined to Allantonectria Earle and Pleonectria and that two species with muriform ascospores included by Seeler (1940) in Thyronectria belong to Nectria s.str., namely N. antarctica and N. pseudotrichia. These latter species have sporodochial or synnematous asexual morphs, while Hirooka et al. (2012) characterised Pleonectria as having pycnidial asexual morphs, although this was not present in all species. Pleonectria now contains also species devoid of longitudinal septa in their ascospores, as had also been determined by Jaklitsch & Voglmayr (2011) using molecular phylogeny.
We have occasionally seen persistent filiform hamathecial threads in perithecial mounts of Pleonectria coryli, P. lamyi or P. pyrrhochlora, just as had been described for Thyronectria patavina. This is why we started to investigate those genera that were relegated to the Thyridiaceae by Rossman et al. (1999) such as Mattirolia. An important species in this context is Pleonectria pyrrhochlora, below recognised as Thyronectria rhodochlora, also considered an earlier name for T. patavina, which led us to conclude that Thyronectria is the correct genus for these species. Another important step in this work was the collection of Mattirolia roseovirens on its original host genus Laburnum in its original region. In conclusion we synonymise Mattirola, Pleonectria and Thyronectroidea with Thyronectria below.
MATERIALS AND METHODS
Isolates and specimens
All isolates used in this study originated from ascospores or conidia of fresh specimens. Numbers of strains including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. Strain acronyms other than those of official culture collections (ATCC, CBS, MAFF) are used here primarily as strain identifiers throughout the work. Representative isolates have been deposited at the CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS). Details of the specimens used for morphological investigations are listed in the Taxonomy section under the respective descriptions. Herbarium acronyms are according to Thiers (2014). Freshly collected specimens have been deposited in the Herbarium of the Institute of Botany, University of Vienna (WU).
Table 1.
Isolates and accession numbers used in the phylogenetic analyses. Isolates/sequences in bold were isolated/sequenced in the present study.
| Species | Isolate No. | Herbarium No. | Substrate/Host | Country | GenBank accession numbers |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| act | ITS | LSU | rpb1 | rpb2 | tef1 | tub | |||||
| Allantonectria miltina | CBS 121121 | BPI 878442 | Agave americana | Italy | HM484514 | HM484547 | HM484572 | HM484587 | – | HM484524 | HM484609 |
| Camarops ustulinoides | AFTOL-ID 72, D.E.H. 2164 | DQ470941 | DQ471121 | DQ470882 | DQ471050 | ||||||
| Cosmospora coccinea | CBS 114050 | BPI 802729 | Inonotus nodulosus | Germany | GQ505990 | GQ506020 | – | HM484515 | |||
| Cyanonectria cyanostoma | CBS 101734 | BPI 748307 | Buxus sempervirens | France | FJ474081 | GQ506017 | – | HM484535 | |||
| Diaporthe eres | CBS 109767 | BPI 748435 | Acer campestre | Austria | AF408350 | DQ471161 | DQ470919 | DQ479931 | |||
| Diatrype disciformis | CBS 197.49 | Alnus sp. | Netherlands | DQ470964 | DQ471158 | DQ470915 | DQ471085 | ||||
| Gnomonia gnomon | CBS 199.53 | Corylus avellana | Italy | AF408361 | DQ471167 | DQ470922 | DQ471094 | ||||
| Nectria antarctica | ATCC 204178, CBS 115033 | BPI 746217 | Berberis aquifolium | USA | HM484560 | HM484575 | – | HM484516 | |||
| N. asiatica | MAFF 241439 | BPI 879972 | unid. dead bark | Japan | HM484505 | HM484701 | HM484563 | – | JQ014140 | – | HM484604 |
| N. balansae | CBS 123351 | BPI 878477 | Coronilla sp. | France | GQ505996 | GQ506026 | – | HM484525 | |||
| N. cinnabarina | CBS 125165 | BPI 879981 | Aesculus sp. | France | HM484503 | HM484548 | HM484562 | HM484577 | JQ014125 | HM484527 | HM484606 |
| N. dematiosa | CBS 126570 | BPI 749337 | unid. dead bark | USA | HM484502a | HM484557 | HM484561 | HM484576 | JQ014144 | HM484534 | HM484603 |
| N. nigrescens | CBS 125148 | BPI 871083 | unid. dead twigs | USA | HM484618 | HM484707 | HM484720 | HM484781 | JQ014123 | HM484672 | HM484806 |
| N. pseudotrichia | CBS 652.83 | PDD 7908 | unid. dead bark | Venezuela | JF832703 | JF832782 | – | JF832528 | |||
| Ophiostoma piliferum | CBS 158.74 | unid. wood | Chile | DQ470955 | DQ471147 | DQ470905 | DQ471074 | ||||
| Papulosa amerospora | AFTOL-ID 748, J.K. 5547F | DQ470950 | DQ471143 | DQ470901 | DQ471069 | ||||||
| Pseudonectria pachysandricola | CBS 128674 | BPI 879936 | Pachysandra sp. | USA | JF832715 | JF832791 | – | JF832544 | |||
| Rodentomyces reticulatus | CBS 128675 | Rodent dung | Italy | JF832717 | – | – | JF832543 | ||||
| Rugonectria rugulosa | CBS 129158 | BPI 881070 | unid. dead bark | USA | JF832761 | JF832836 | – | JF832545 | |||
| Thelonectria westlandica | CBS 112464 | Dacrydium cupressinum | New Zealand | GQ505987 | GQ506015 | – | HM484533 | ||||
| Thyridium vestitum | CBS 113027 | BPI 842278 | Acer pseudoplatanaus | Austria | AY544671 | DQ471129 | DQ470890 | DQ471058 | |||
| Thyronectria aquifolii | CBS 307.34 | BPI 550125 | Ilex aquifolium | UK | JF832444 | JF832597 | JF832718 | JF832792 | – | JF832548 | JF832842 |
| NAK, CBS 125027 | WU 30360 | Ilex aquifolium | UK | KJ570663 | HM534891 | HM534891 | KJ570715 | HM534881 | HM534870 | KJ570638 | |
| T. asturiensis | MA3, CBS 136000 | WU 32124 | Quercus ilex | Spain | KJ570664 | KJ570690 | KJ570690 | KJ570716 | KJ570741 | KJ570760 | KJ570639 |
| T. aurigera | CBS 109874 | BPI 841465 | Fraxinus excelsior | France | HM484511 | HM484551 | HM484573 | HM484586 | – | HM484521 | HM484600 |
| T. austroamericana | CBS 125134 | BPI 746395, NCSU | Gleditsia triacanthos | USA | JF832513 | JF832654 | JF832759 | JF832834 | – | JF832587 | JF832881 |
| CBS 125135 | NCSU | Robinia pseudoacacia | USA | JF832514 | JF832655 | JF832760 | JF832835 | – | JF832588 | JF832882 | |
| CBS 126114 | BPI 746395 | Gleditsia triacanthos | USA | GQ505960 | HM484555 | GQ505988 | GQ506016 | – | HM484520 | HM484597 | |
| GG | WU 32664 | Gymnocladus dioicus | Austria | KJ570665 | KJ570691 | KJ570691 | KJ570717 | KJ570742 | KJ570761 | KJ570640 | |
| T. balsamea | CBS 125132 | BPI 746322 | Abies fraseri | USA | JF832453 | JF832598 | JF832719 | JF832800 | JQ014122 | JF832556 | JF832846 |
| CBS 129159 | BPI 881047 | Abies balsamea | USA | JF832456 | JF832601 | JF832721 | JF832803 | – | JF832557 | – | |
| CBS 125136 | NCSU | Abies fraseri | USA | JF832455 | JF832600 | JF832727 | JF832804 | – | JF832559 | JF832847 | |
| CBS 129160 | BPI 881050 | Abies balsamea | USA | JF832457 | JF832667 | JF832731 | JF832807 | – | JF832558 | JF832851 | |
| CBS 129429 | BPI 881048 | Abies balsamea | USA | JF832458 | JF832610 | JF832730 | JF832802 | – | JF832562 | JF832850 | |
| CBS 125137 | NCSU | Abies fraseri | USA | JF832454 | JF832599 | JF832729 | JF832805 | JQ014142 | JF832561 | JF832849 | |
| CBS 129428 | BPI 881049 | Abies balsamea | USA | JF832460 | JF832668 | JF832732 | JF832801 | – | JF832560 | JF832848 | |
| T. berolinensis | CBS 126112 | BPI 746346 | Ribes rubrum | Austria | HM484510 | HM484543 | HM484568 | HM484583 | – | HM484517 | HM484594 |
| CBS 128980 | HB7896A | Ribes nigrum | Mongolia | JF832479 | JF832623 | JF832750 | JF832829 | – | JF832584 | JF832875 | |
| NB, CBS 127382 | WU 30361 | Ribes sanguineum | Austria | KJ570666 | HM534893 | HM534893 | KJ570718 | HM534883 | HM534872 | KJ570641 | |
| T. boothii | CBS 128977 | BPI 881052 | Picea abies | Slovakia | JF832475 | JF832617 | JF832755 | JF832796 | – | JF832552 | JF832871 |
| T. caudata | NL2, CBS 136003 | WU 32130 | Berberis cretica | Greece | KJ570667 | KJ570692 | KJ570692 | KJ570719 | KJ570743 | KJ570762 | KJ570642 |
| NCA | WU 33429 | Berberis hispanica | Spain | KM225674 | KM225679 | KM225679 | KM225685 | KM225690 | KM225692 | KM225697 | |
| NCA1 | WU 33428 | Berberis hispanica | Spain | KM225675 | KM225680 | KM225680 | KM225686 | KM225691 | KM225693 | KM225698 | |
| T. coryli | CBS 129358 | BPI 881053, C.L.L. 651 | Corylus avellana | France | JF832476 | JF832672 | JF832740 | JF832797 | – | JF832553 | JF832872 |
| CBS 115619 | BPI 746347 | Viburnum lantana | Austria | JF832477 | JF832618 | JF832741 | JF832798 | – | JF832554 | JF832873 | |
| CBS 129156 | BPI 880697 | Rhus copallinum | USA | HM484509 | HM484539 | HM484566 | HM484581 | – | HM484536 | HM484596 | |
| CBS 129744 | BPI 881054 | Celastrus orbiculatus | USA | JF832478 | JF832619 | JF832742 | JF832799 | – | JF832555 | JF832874 | |
| NCP, CBS 127384 | WU30362 | Pyrus communis | Austria | KJ570668 | HM534895 | HM534895 | KJ570720 | HM534885 | HM534874 | KJ570643 | |
| NeCo1, CBS 137264 | WU 32129 | Corylus avellana | Austria | KJ570669 | KJ570693 | KJ570693 | KJ570721 | KJ570744 | KJ570763 | KJ570644 | |
| T. cucurbitula | CBS 301.75 | Pinus nigra | France | JF832461 | JF832621 | JF832720 | JF832808 | – | JF832563 | JF832854 | |
| CBS 259.58 | Pinus sylvestris | Netherlands | GQ505974 | HM484541 | GQ505998 | GQ506028 | JQ014131 | HM484530 | HM484592 | ||
| CBS 541.70 | Dead twig in witch‘s broom | Netherlands | JF832463 | JF832602 | JF832722 | JF832809 | – | JF832565 | JF832856 | ||
| CBS 125130 | BPI 746348 | Pinus sylvestris | Austria | JF832464 | JF832603 | JF832723 | JF832811 | – | JF832564 | JF832855 | |
| CBS 178.73 | Pinus sylvestris | Netherlands | JF832462 | JF832607 | JF832733 | JF832810 | JQ014134 | JF832566 | JF832857 | ||
| T. ilicicola | CBS 125147 | BPI 880698 | Ilex aquifolium | UK | HM484506 | HM484538 | HM484565 | HM484579 | – | HM484522 | HM484590 |
| CBS 125170 | BPI 881055, C.L.L. 7159 | Ilex aquifolium | France | JF832445 | JF832625 | JF832756 | JF832793 | – | JF832549 | JF832843 | |
| CBS 125171 | BPI 881055, C.L.L. 7159 | Ilex aquifolium | France | JF832446 | JF832626 | JF832758 | JF832794 | – | JF832550 | JF832844 | |
| CBS 128978 | BPI 879857, C.L.L. 7184 | Ilex aquifolium | France | JF832447 | JF832673 | JF832757 | JF832795 | – | JF832551 | JF832845 | |
| T. lamyi | CBS 115034 | BPI 746349 | Berberis vulgaris | Austria | HM484507 | HM484544 | HM484569 | HM484582 | – | HM484518 | HM484593 |
| NL, CBS 127385 | WU 30363 | Berberis thunbergii | Austria | KJ570670 | HM534898 | HM534898 | – | HM534888 | HM534877 | KJ570645 | |
| NL1 | WU 32141 | Berberis candidula | Austria | KJ570671 | KJ570694 | KJ570694 | KJ570722 | KJ570745 | KJ570764 | KJ570646 | |
| NL3, CBS 137263 | WU 32159 | Berberis vulgaris | Austria | KJ570672 | KJ570695 | KJ570695 | KJ570723 | KJ570746 | KJ570765 | KJ570647 | |
| NL4 | WU 32165 | Berberis hispanica | Spain | – | KJ570696 | KJ570696 | – | – | – | – | |
| NL5 | WU 32166 | Berberis hispanica | Spain | – | KJ570697 | KJ570697 | – | – | – | – | |
| NL6 | WU 32167 | Berberis hispanica | Spain | – | KJ570698 | KJ570698 | – | – | – | – | |
| NL7 | WU 32169 | Berberis hispanica | Spain | – | KM225681 | KM225681 | – | – | – | – | |
| T. obscura | TT, CBS 136923 | WU 32142 | Tamarix tetrandra | Austria | KJ570673 | KJ570699 | KJ570699 | KJ570724 | KJ570747 | KJ570766 | – |
| TT1 | WU 32143 | Tamarix parviflora | Austria | KJ570674 | KJ570700 | KJ570700 | KJ570725 | KJ570748 | KJ570767 | – | |
| T. okinawensis | CBS 129369, MAFF 241410 | BPI 881058, TUA-TPP-h92 | Castanopsis sp. | Japan | JF832451 | JF832674 | JF832751 | JF832827 | – | JF832585 | JF832878 |
| CBS 129745 | TUA-TPP-h93 | Castanopsis sp. | Japan | JF832452 | JF832675 | JF832752 | JF832828 | – | JF832586 | JF832879 | |
| T. pinicola | MAFF 241458 | BPI 881061, TUA-TPP-h543 | Pinus koraiensis | Japan | JF832469 | JF832676 | JF832748 | JF832823 | – | JF832572 | JF832862 |
| CBS 125166 | BPI 881059 | Pinus sylvestris | Germany | HM484508 | HM484540 | HM484567 | HM484580 | – | HM484528 | HM484591 | |
| CBS 242.30 | Pinus sylvestris | Russia | – | JF832615 | JF832747 | JF832822 | – | JF832573 | JF832863 | ||
| CBS 125167 | BPI 881060 | Pinus sylvestris | Germany | JF832470 | JF832616 | JF832749 | JF832824 | – | JF832574 | JF832864 | |
| T. quercicola | CBS 128976 | BPI 871328 | Quercus ilex ssp. rotundifolia | Spain | JF832450 | JF832624 | JF832743 | JF832831 | – | JF832581 | JF832880 |
| T. rhodochlora | CBS 125131 | BPI 746398 | Acer campestre | Austria | HM484512 | HM484545 | HM484570 | HM484584 | – | HM484519 | HM484598 |
| NP, CBS 136004 | WU 31653 | Acer opalus | France | KJ570675 | KJ570701 | KJ570701 | KJ570726 | KJ570749 | KJ570768 | KJ570648 | |
| NP1 | WU 31654 | Acer campestre | Italy | KJ570676 | KJ570702 | KJ570702 | KJ570727 | KJ570750 | KJ570769 | KJ570649 | |
| NP2, CBS 136005 | WU 31655 | Acer campestre | Austria | KJ570677 | KJ570703 | KJ570703 | KJ570728 | KJ570751 | KJ570770 | KJ570650 | |
| NP3, CBS 136006 | WU 31656 | Acer campestre | Austria | KJ570678 | KJ570704 | KJ570704 | KJ570729 | – | KJ570771 | KJ570651 | |
| NP4 | WU 32149 | Acer campestre | Austria | KJ570679 | KJ570705 | KJ570705 | KJ570730 | KJ570752 | KJ570772 | KJ570652 | |
| NP5 | WU 32150 | Koelreuteria paniculata | Austria | KJ570680 | KJ570706 | KJ570706 | KJ570731 | – | KJ570773 | KJ570653 | |
| NP7 | WU 32152 | Prunus tenella | Austria | KJ570681 | KJ570707 | KJ570707 | KJ570732 | – | KJ570774 | KJ570654 | |
| NP8 | WU 33425a | Ulmus minor | Austria | KM225676 | KM225682 | KM225682 | KM225687 | – | KM225694 | KM225699 | |
| NP9 | WU 33425b | Corylus avellana | Austria | KM225677 | KM225683 | KM225683 | KM225688 | – | KM225695 | KM225700 | |
| T. rosellinii | MAFF 241459, NITE 102242 | BPI 881062 | unid. dead twigs | Japan | JF832471 | JF832611 | JF832736 | JF832816 | – | JF832576 | JF832866 |
| CBS 128975 | BPI 747280 | Abies fraseri | USA | JF832472 | JF832612 | JF832737 | JF832817 | – | – | JF832868 | |
| CBS 129427 | BPI 881065 | Abies balsamea | USA | JF832473 | JF832613 | JF832738 | JF832819 | – | JF832577 | JF832869 | |
| CBS 129162 | BPI 881066 | Abies balsamea | USA | JF832474 | JF832614 | JF832739 | JF832820 | – | JF832578 | JF832870 | |
| T. roseovirens | MA | WU 32153 | Retama sphaerocarpa | Spain | KJ570682 | KJ570708 | KJ570708 | KJ570733 | KJ570753 | KJ570775 | KJ570655 |
| MA1, CBS 135999 | WU 32154 | Laburnum alpinum | Italy | KJ570683 | KJ570709 | KJ570709 | KJ570734 | KJ570754 | KJ570776 | KJ570656 | |
| MA2 | WU 32155 | Laburnum alpinum | Italy | KJ570684 | KJ570710 | KJ570710 | KJ570735 | KJ570755 | KJ570777 | KJ570657 | |
| MA4 | WU 32156 | Genista florida | Spain | KJ570685 | KJ570711 | KJ570711 | KJ570736 | KJ570756 | KJ570778 | KJ570658 | |
| MA5 (from ascospore) | WU 32157 | Genista florida | Spain | KJ570686 | KJ570712 | KJ570712 | KJ570737 | KJ570757 | KJ570779 | KJ570659 | |
| MA5a (from conidium), CBS 136001 | WU 32157 | Genista florida | Spain | KJ570687 | KJ570713 | KJ570713 | KJ570738 | KJ570758 | KJ570780 | KJ570660 | |
| MA6, CBS 136002 | WU 32158 | Genista florida | Spain | KJ570688 | KJ570714 | KJ570714 | KJ570739 | KJ570759 | KJ570781 | KJ570661 | |
| T. sinopica | CBS 128981 | C.L.L. 9237 | Hedera sp. | France | JF832448 | JF832622 | JF832744 | JF832825 | – | JF832582 | JF832876 |
| CBS 125169 | BPI 881067, C.L.L. 7156 | Hedera helix | France | JF832449 | JF832620 | JF832745 | JF832826 | – | JF832583 | JF832877 | |
| CBS 462.83 | CBS H-19479, CBS H-19485 | Hedera helix | Netherlands | GQ505973 | HM484542 | GQ506001 | GQ506031 | – | HM484531 | HM484595 | |
| NS, CBS 127386 | WU 30364 | Hedera helix | Austria | KJ570689 | HM534900 | HM534900 | KJ570740 | HM534890 | HM534879 | KJ570662 | |
| T. strobi | CBS 102036 | BPI 1107115 | Pinus strobus | USA | JF832465 | JF832604 | JF832734 | JF832812 | – | JF832567 | JF832858 |
| CBS 129363 | BPI 1112876 | Pinus strobus | USA | JF832468 | JF832608 | JF832724 | JF832815 | – | JF832568 | JF832860 | |
| CBS 125107 | NY | Pinus strobus | USA | JF832467 | JF832605 | JF832725 | JF832813 | – | JF832569 | JF832861 | |
| CBS 125122 | NY | Pinus strobus | USA | JF832466 | JF832606 | JF832726 | JF832814 | – | JF832570 | JF832859 | |
| T. cf. virens | A.R. 4558, Y.H. 08-11 | BPI 881068, C.L.L. 7181 | Acer sp. | France | JF832509 | JF832677 | JF832754 | JF832832 | – | JF832589 | JF832883 |
| NP10 | WU 33426 | Ostrya carpinifolia | France | KM225678 | KM225684 | KM225684 | KM225689 | – | KM225696 | KM225701 | |
| T. zanthoxyli | CBS 129157 | BPI 881069 | unid. dead bark | USA | JF832510 | JF832627 | JF832753 | JF832833 | – | JF832590 | JF832884 |
| CBS 124736 | C.L.L. 7132 | Crataegus sp. | France | JF832511 | – | – | – | – | JF832591 | JF832885 | |
| CBS 126113 | BPI 878445 | Crataegus sp. | France | HM484513 | HM484546 | HM484571 | HM484585 | – | HM484523 | HM484599 | |
| Xylaria hypoxylon | AFTOL-ID 51, OSC 100004 | AY544648 | DQ471114 | DQ470878 | DQ471042 | ||||||
A.R.: Amy Y. Rossman, USDA-ARS MD USA; ATCC: American Type Culture Collection, Manassas, VA, USA; BPI: U.S. National Fungus Collections USDA-ARS MD USA; CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; C.L.L.: Christian Lechat, Ascofrance, Villiers en Bois, France.; D.E.H.: Don E. Hemmes; G.J.S.: Gary J. Samuels, USDA-ARS MD USA; J.K.: Jan Kohlmeyer; MAFF: MAFF Genebank, National Institute of Agrobiological Sciences, Ibaraki, Japan; NITE: NBRC, National Institute of Technology and Evaluation, Chiba, Japan; NCSU: The Mycological Herbarium, North Carolina State University, NC, USA; NY: William and Lynda Steere Herbarium, The New York Botanical Garden, NY, USA; OSC: Oregon State University Herbarium, OR, USA; PDD: New Zealand Fungus Herbarium, Auckland, New Zealand; TUA-TPP-h: Yuuri Hirooka, Tropical Plant Protection Lab Herbarium, Tokyo University of Agriculture, Tokyo Japan; WU: Herbarium of the University of Vienna, Austria; Y.H.: Yuuri Hirooka, USDA-ARS MD USA.
Culture preparation, growth rate determination and phenotype analysis
Cultures were prepared and maintained as described previously (Jaklitsch 2009) except that CMD (CMA: Sigma, St Louis, Missouri; supplemented with 2 % (w/v) D(+)-glucose-monohydrate) or 2 % malt extract agar (MEA; 2 % w/v malt extract, 2 % w/v agar-agar; Merck, Darmstadt, Germany) was used as the isolation medium. Cultures used for the study of asexual morph micro-morphology were grown on CMD or 2 % MEA (or potato dextrose agar (PDA, 39 g/L; Merck, Darmstadt, Germany) where noted) at room temperature (RT), defined here as 22 ± 3 °C, or at 25 °C under alternating 12 h cool daylight and 12 h darkness. Sectioning with a freezing microtome was carried out after short rehydration and treatment with 3 % KOH at 8–12 μm as described previously (Jaklitsch & Voglmayr 2011). Microscopic observations were generally made in de-ionised water or 3 % KOH, lactic acid or 50 % glycerol where noted. Morphological analyses of microscopic characters were carried out as described earlier (Jaklitsch 2009). Data were gathered using a Nikon Coolpix 4500 or a Nikon DS-U2 digital camera and measured by using the NIS-Elements D v. 3.0 software. Methods of microscopy included stereomicroscopy using a Nikon SMZ 1500 and Nomarski differential interference contrast (DIC) using the compound microscope Nikon Eclipse E600. For certain images of stromata the stacking software Zerene Stacker v. 1.04 (Zerene Systems LLC, Richland, WA, USA) was used. Measurements are reported as maxima and minima in parentheses and the mean plus and minus the standard deviation of a number of measurements given in parentheses. The colour term rosy is used for a certain range of pinkish colours as exemplified by ascospores of T. rhodochlora (Fig. 4) and conidiation structures of T. roseovirens (Fig. 12h,i).
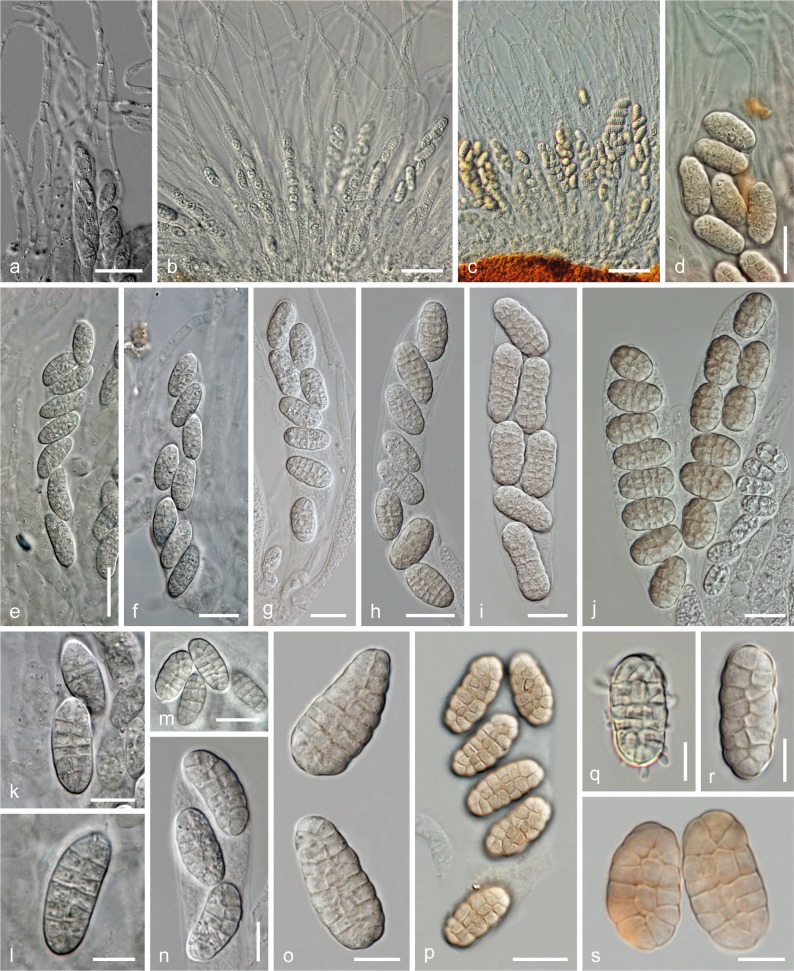
Fig. 4.
Thyronectria rhodochlora, centrum characteristics. a–d. Apical paraphyses among immature (a, b) and mature (c, d) asci in water; e–j. asci with ascospores; k–s. ascospores (q. overmature, producing conidia; all in water except p (in water after swelling in 3 % KOH) and s. (in lactic acid in a microtome section)). a, e, f, k, l: T. patavina holotype (PAD); b: WU 32150 (NP5); c, h, i, o, s: epitype WU 31656 (NP3); d, p: T. rhodochlora isolectotype (K); g, n, r: WU 31654 (NP1); j: WU 32152 (NP7); m: T. rhodochlora lectotype (PC)); q. WU 33425a (NP8). — Scale bars: a, e, h = 20 μm; b = 30 μm; c = 50 μm; d, f, g, i, j, m, p = 15 μm; k, n, o = 10 μm; l, q–s = 7 μm.
Fig. 12.
Thyronectria roseovirens, ascomata and stromata (h, i, k. showing rosy asexual morph). Note direct growth on the host fungus Cucurbitaria laburni in a (a–d, o: WU 32154 (MA1; o. in 3 % KOH); e, k: WU 32153 (MA); f, g: lectotype (FH); h, i, m: WU 32156 (MA4); j: WU 32157 (MA5); l: WU 32155 (MA2); n: WU 32158 (MA6)). — Scale bars: a, d, g = 0.3 mm; b, c, e, f, h–o = 0.5 mm.
DNA extraction and sequencing methods
The extraction of genomic DNA was performed as reported previously (Voglmayr & Jaklitsch 2011, Jaklitsch et al. 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany) or the modified CTAB method of Riethmüller et al. (2002). Seven loci were amplified and sequenced of which six correspond to those included in Hirooka et al. (2012): a c. 700 bp fragment of alpha-actin (act) with primers Tact1 and Tact2 (Samuels et al. 2006); the complete internally transcribed spacer region (ITS1-5.8S-ITS2) and a c. 900 bp fragment of the large subunit nuclear ribosomal DNA (nLSU rDNA), amplified and sequenced as a single fragment with primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990); a c. 700 bp fragment of the RNA polymerase II subunit 1 (rpb1) with primers crpb1a and rpb1c (Castlebury et al. 2004); a c. 1.2 kb fragment of the RNA polymerase II subunit 2 (rpb2) with primers fRPB2-5f and fRPB2-7cr (Liu et al. 1999); a c. 1.3 kb fragment of the translation elongation factor 1-alpha (tef1) with primers EF1-728F (Carbone & Kohn 1999) and TEF1LLErev (Jaklitsch et al. 2005); and a c. 1 kb fragment of β-tubulin (tub) with primers Btub-T1 and Btub-T222 (O’Donnell & Cigelnik 1997). PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994) as described in Voglmayr & Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington, UK) with the same primers as in PCR and an automated DNA sequencer (3730xl Genetic Analyzer, Applied Biosystems); in addition, internal primers ITS4 (White et al. 1990) and LR3 (Vilgalys & Hester 1990) were used for sequencing the partial nuSSU-complete ITS-partial nuLSU rDNA region.
Analysis of sequence data
All alignments were produced with the server version of MAFFT (www.ebi.ac.uk/Tools/msa/mafft), checked and refined using Bio-Edit v. 7.0.4.1 (Hall 1999). To investigate the phylogenetic relationships of ‘Mattirolia’ and Thyronectria to Thyridium and Nectriaceae, a multigene matrix comprising LSU, rpb1, rpb2 and tef1 sequences was produced and analysed. In addition to sequences obtained in the current study, representative GenBank sequences were selected from Hirooka et al. (2012) and Spatafora et al. (2006). The resulting combined 4-gene sequence matrix contained 42 taxa and 4 178 alignment positions (825, 779, 1 129 and 1 445 characters from LSU, rpb1, rpb2 and tef1, respectively). According to Spatafora et al. (2006), Diatrype disciformis and Xylaria hypoxylon (Xylariales, Xylariomycetidae) were selected as outgroup. Prior to phylogenetic analyses, the approach of Wiens (1998) was applied to test for significant levels of localised incongruence among the 4-gene partitions, using the level of bootstrap support (Sung et al. 2007). For this, the 70 % maximum parsimony (MP) bootstrap consensus trees calculated for each individual partition, using the same parameters as for the combined analysis given below, were compared. No topological conflicts were observed between these bootstrap trees of the various genes, indicating the absence of significant incongruence and combinability of the four loci (Wiens 1998).
For detailed investigation of phylogenetic relationships within Thyronectria, sequences of the six loci included in Hirooka et al. (2012) were downloaded from GenBank and combined with those generated during the present study; in addition, sequence data for the rpb2 were added when available. Nectria asiatica, N. cinnabarina, N. dematiosa and N. nigrescens were selected as outgroup. The resulting combined sequence matrix contained 6 150 alignment positions from seven genes (630 from act, 512 from ITS, 807 from LSU, 695 from rpb1, 1 187 from rpb2, 1 278 from tef1 and 1 041 from tub). Prior to phylogenetic analyses, the approach of Wiens (1998) was applied to test for significant levels of localised incongruence among the 7-gene partitions as described above. No topological conflicts were observed between the bootstrap trees of the various genes, indicating the absence of significant incongruence and combinability of the seven loci (Wiens 1998).
Maximum parsimony (MP) analyses were performed with PAUP v. 4.0 b10 (Swofford 2002), using 1 000 replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data. The COLLAPSE command was set to NO for the combined 4-gene and to MINBRLEN for the combined 7-gene matrix. Bootstrap analyses with 500 replicates were performed in the same way, but using five rounds of random sequence addition and subsequent branch swapping during each bootstrap replicate; in addition, each replicate was limited to 10 million rearrangements in the analyses of the combined 7-gene matrix.
In ML and Bayesian analyses, substitution model parameters were calculated separately for the different gene regions included in the combined analyses. For ML analyses, 500 fast bootstrap replicates were computed with RAxML (Stamatakis 2006a) as implemented in raxmlGUI v. 1.3 (Silvestro & Michalak 2012) using the GTRCATI substitution model, which efficiently approximates the well-known general time reversible model (GTR; Rodríguez et al. 1990) with gamma-distributed substitution rates, additionally assuming a proportion of invariant sites (GTR+I+G) (Stamatakis 2006b).
Bayesian analyses were performed with the computer program MrBayes (v. 3.2.2; Huelsenbeck & Ronquist 2001). The following substitution models were selected by Modeltest v. 3.6 (Posada & Crandall 1998) under the Akaike Information Criterion: in the 4-gene matrix for all loci the GTR+I+G model; in the 7-gene matrix for tub TRN+G and for the remaining loci the GTR+I+G model. As the TRN+G model could not be implemented in MrBayes, the GTR+I+G model was applied as most similar model for all partitions. Three parallel runs of four incrementally heated, simultaneous Markov chains were performed over 10 million generations, of which every 1 000th tree was sampled in each run. The first 500 trees sampled were discarded, and a 90 % majority rule consensus of the remaining trees was computed to obtain posterior probabilities (PP). To test convergence of runs, the results were analysed using AWTY (Nylander et al. 2008); no indication of lack of convergence was detected.
RESULTS
Molecular phylogeny
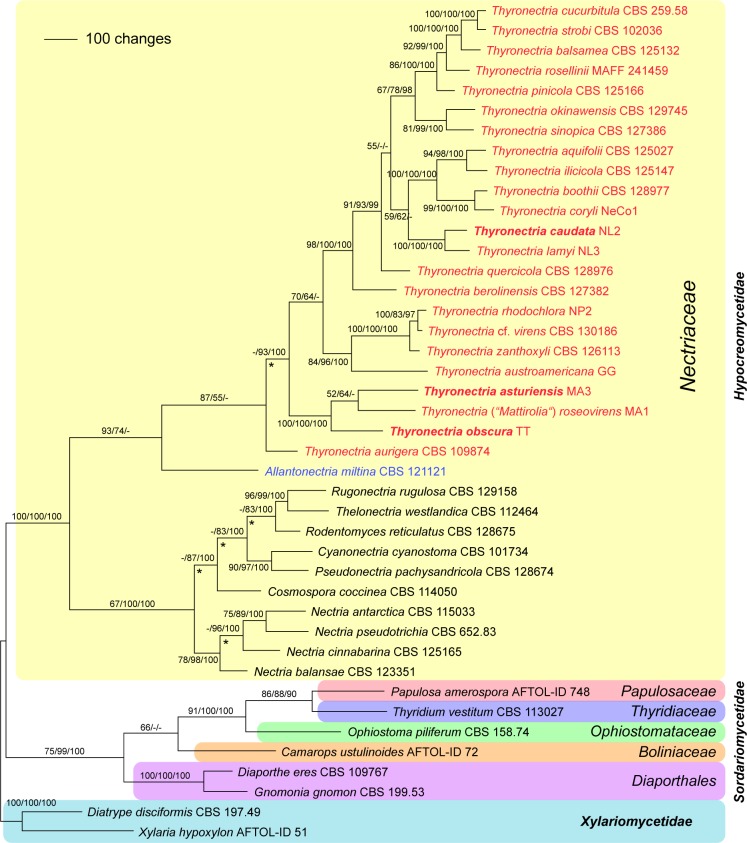
Of the 4 178 characters included in the combined 4-gene matrix, 1 573 were parsimony informative (161 in LSU, 403 in rpb1, 524 in rpb2 and 485 in tef1). MP analyses revealed four MP trees with a score of 9 282, one of which is shown as Fig. 1. The four MP trees differed in the nodes lacking MP bootstrap support (Fig. 1); i.e. the position of Thyronectria aurigera which is either sister to all other Thyronectria species or sister to the T. asturiensis/T. obscura/T. roseovirens clade, and in some topological differences within the residual Nectriaceae clade. Tree topologies of the Bayesian analyses were fully congruent with the MP tree. The three Bayesian runs revealed almost identical posterior probabilities. MP and ML bootstrap support above 50 % and Bayesian posterior probabilities above 90 % are given in Fig. 1 in this order above or below the branches.
Fig. 1.
Phylogram showing one of four MP trees 9 282 steps long revealed by PAUP from an analysis of the combined 4-gene (LSU, rpb1, rpb2, tef1) matrix of selected Xylariomycetidae, Sordariomycetidae and Nectriacae, showing the phylogenetic position of Thyridium, Thyronectria and Mattirolia (given as Thyronectria roseovirens in the tree). MP and ML bootstrap support above 50 % and Bayesian posterior probabilities above 90 % are given above or below the branches. Strain/culture designations are given following the taxon names; new species are marked in bold italics. Nodes marked by an asterisk (*) collapsed in the strict consensus of the four MP trees.
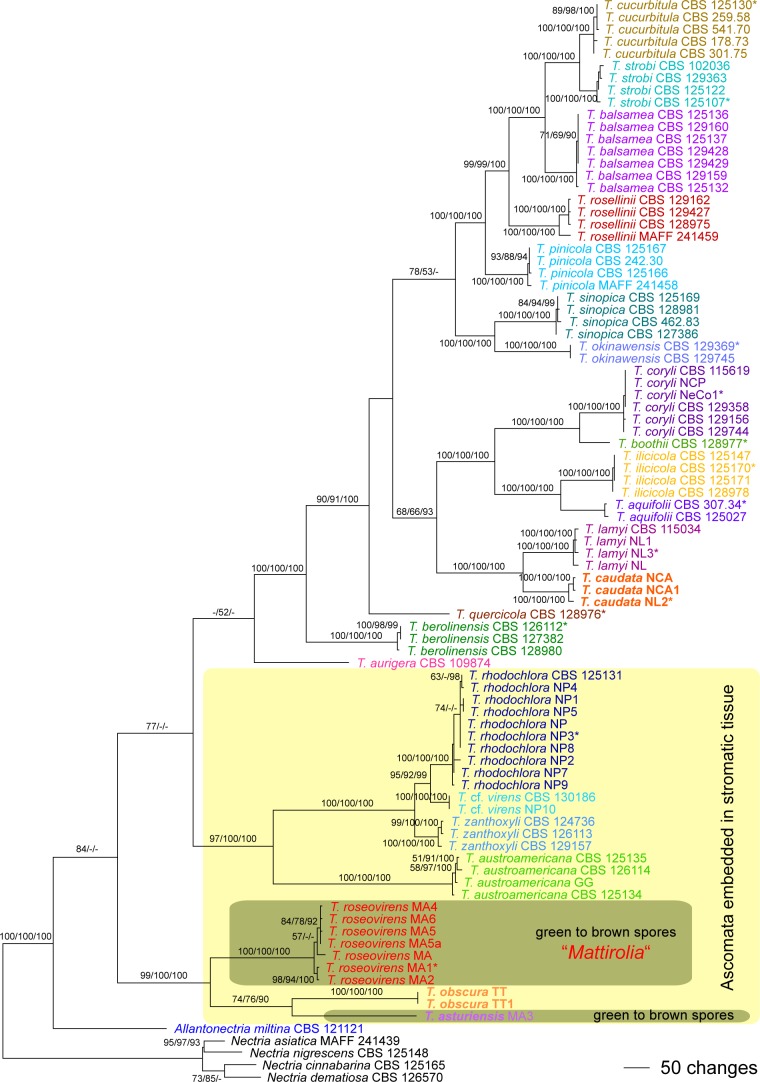
Of the 6 150 characters included in the combined 7-gene matrix, 1 794 were parsimony informative (159 in act, 102 in ITS, 78 in LSU, 280 in rpb1, 440 in rpb2, 419 in tef1 and 316 in tub). MP analyses revealed 216 MP trees with a score of 6 654, one of which is shown as Fig. 2. All MP trees were identical except for minor topological differences within the same species (data not shown). Tree topologies of the Bayesian analyses were fully congruent with the MP tree. The three Bayesian runs revealed almost identical posterior probabilities. MP and ML bootstrap support above 50 % and Bayesian posterior probabilities above 90 % are given in Fig. 2 in this order above or below the branches.
Fig. 2.
Phylogram showing one of 216 MP trees 6 654 steps long revealed by PAUP from an analysis of the combined 7-gene (act, ITS, LSU, rpb1, rpb2, tef1, tub) matrix of Allantonectria and Thyronectria, with four species of Nectria s.str. selected as outgroup. MP and ML bootstrap support above 50 % and Bayesian posterior probabilities above 90 % are given above or below the branches. Strain/culture designations are given following the taxon names; new species are formatted in bold; holo-, neo- or epitype strains/specimens are marked by an asterisk (*).
As shown in Fig. 1, molecular data confirm the placement of Thyronectria and ‘Mattirolia’ (T. roseovirens) within Nectriaceae (Hypocreomycetidae), whereas Thyridium vestitum is placed as sister to Papulosaceae within Sordariomycetidae. Within Thyronectria, tree topologies agree well with Hirooka et al. (2012) except for slightly different positions of T. aurigera in the 7-gene tree and of T. lamyi and T. quercicola in both trees (Fig. 1, 2).
Thyronectria roseovirens, the type species of Mattirolia, and the two newly described T. asturiensis and T. obscura form a highly supported clade at near basal (Fig. 1) or basal (Fig. 2) position within Thyronectria (Fig. 1, 2). The third new species, T. caudata, originally described as a variety of T. lamyi, is sister species of T. lamyi with maximum support in all analyses (Fig. 1, 2).
Taxonomy
Available generic names for the monophylum treated here are Aponectria (Sacc.) Sacc., Chilonectria Sacc., Mattirolia Berl. & Bres., Pleonectria Sacc., Scoleconectria Seaver, Thyronectria Sacc. and Thyronectroidea Seaver. The oldest of these names is Thyronectria. The original species Thyronectria patavina Sacc. (1875b), on which Saccardo based his genus, was determined to be a synonym of Sphaeria rhodochlora Mont. (1834), which is older than T. patavina. Therefore the correct epithet of the type species of Thyronectria is T. rhodochlora.
Thyronectria Sacc., Grevillea 4: 21. 1875
= Pleonectria Sacc., Mycoth. Veneta no. 688. 1876.
= Chilonectria Sacc., Michelia 1: 279. 1878.
= Nectria subg. Aponectria Sacc., Michelia 1: 296. 1878.
≡ Aponectria (Sacc.) Sacc., Syll. Fung. 2: 516. 1883.
= Mattirolia Berl. & Bres., Micromycet. Trident.: 55. 1889.
= Scoleconectria Seaver, Mycologia 1: 197. 1909.
= Thyronectroidea Seaver, Mycologia 1: 206. 1909.
Type species. Thyronectria rhodochlora (Mont.) Seeler.
Stromata immersed-erumpent from bark, uni- to multiperitheciate, scattered, aggregated in groups or compact and pulvinate; stromatic tissue soft, light coloured, surrounding individual ascomata or formed as a hypostroma, pseudoparenchymatous or prosenchymatous or both, KOH- or KOH+, typically upper surface covered by KOH- yellow-green amorphous scurf. Ascomata perithecial, immersed in a stroma or superficial and typically densely aggregated on a hypostroma immersed below the bark epidermis, variously shaped, mostly globose to flask-shaped, often collapsed cupulate when dry; apex (ostiolar region) obtuse, often black when mature. Peridium pseudoparenchymatous, consisting of 2–4 layers, the pigmented outer layer(s) in section subhyaline, yellow, orange, red or light brown, when dry yellow-orange, shades of red to brown or black, often distinctly thickened around the ostiole; KOH- or KOH+. Hamathecium present as periphyses in the ostiole and as apical paraphyses, branched and anastomosing, descending from an apical cushion to the bases of asci, typically present at maturity, cylindrical or as submoniliform bands. Asci unitunicate, oblong or clavate, with undifferentiated apex, containing 8 uni- or biseriate ascospores. Ascospores ellipsoid, oblong, fusiform, globose, clavate or vermiform, hyaline, yellowish, rosy, green or brown, with eusepta and/or distosepta becoming indistinct in KOH, 1- to several-septate or muriform, smooth or striate, sometimes budding in the ascus to produce oblong to allantoid, 1-celled, hyaline, ascoconidia.
Asexual morph on natural substrates — When present, effuse, conidial masses forming on white mycelium and conidiophores or pycnidial or both. Pycnidia occurring together with perithecia on the same hypostroma or separately, solitary or aggregated in groups, erumpent-superficial, subglobose to irregularly discoid to cupulate or elongate and erect, rosy, orange, red, violaceous brown to nearly black, KOH+ or KOH-. Sterile hyphae sometimes present inside the pycnidium. Conidiophores densely packed, simple, irregularly or verticillately branched; conidia formed on minute pegs or cylindrical to subulate phialides, conidial formation enteroblastic. Conidia hyaline, oblong, ellipsoid or (sub)allantoid, 1-celled.
Asexual morph in culture — Conidia formed on minute pegs produced on hyphae in the colony above the agar surface or, particularly on CMD, submerged in agar; conidia also formed on pegs and lageniform to ampulliform phialides produced on simple, unbranched or branched, sometimes verticillate conidiophores or formed in pycnidia on mostly lageniform to subulate phialides on densely aggregated, simple, asymmetrically or verticillately branched, often shrub- or fan-shaped conidiophores. Conidia oblong, ellipsoid, cylindrical or allantoid, hyaline, 0- (rarely 1- or 2-)septate, smooth.
Habitat — On dead corticated twigs or branches of woody plants and/or fungi colonizing them.
Distribution — Mostly north and south temperate, sometimes also found in subtropical regions of Asia, the Caribbean, Central and South America.
Thyronectria rhodochlora (Mont.) Seeler, J. Arnold Arbor. 21: 455. 1940. — Fig. 3, 4, 5
Fig. 3.
Thyronectria rhodochlora, stromata and ascomata. a–h, j–m. Dry stromata in surface view (a. habit; b. with pycnidia of a Diplodia sp.); i. stromata in 3 % KOH after rehydration; n. perithecium in vertical section; o. lower lateral portion of a perithecium showing asci and apical paraphyses in section; p–r. peridium in section (q. basal region; r. ostiolar region with periphyses and scurf); s. stroma hyphae (n–p, r: in 50 % glycerol; q, s: in lactic acid). a, e, i, n–s: epitype WU 31656 (NP3); b, c, g: WU 32152 (NP7); d, f, h: WU 31654 (NP1); j, k: lectotype (PC); l: isolectotype (K); m: holotype of T. patavina (PAD). — Scale bars: a, m = 1 mm; b, e, h, i = 0.4 mm; c, d, f, j–l = 0.3 mm; g = 0.7 mm; n = 100 μm; o, q = 30 μm; p, r, s = 15 μm.
Fig. 5.
a–l: Thyronectria rhodochlora, asexual morph. a, b. Pycnidia (MEA, 15 °C, 65 d); c–h. conidiophores and phialides (c, d, h. from pycnidium on MEA, 15 °C, 65 d; c. shrub-like; e–g. from effuse conidiation, showing pegs and phialides; MEA, RT, 3 d); i–l. conidia (i, j: MEA, RT, 3 d; k, l: from pycnidium on MEA, 15 °C, 65 d) (all in water except h (in 3 % KOH)). a–d, h, k, l: NP7; e–g, i, j: NP. — m–s. Thyronectria virens (isolectotype BPI 631193). m–o. Ascomata in bark (o. old open ascoma attached to the side of a Diplodia conidioma); p–s. ascospores. — t–w. Ascospores of Thyronectria cf. virens (WU 33426; u. in ascus, note apical paraphyses). — x–aa. Thyronectria zanthoxyli. x, y. Stromata; z, aa. ascospores. x, y, aa. lectotype NYS 3611; z. isolectotype NYS 3438. — Scale bars: a, m, n, x = 0.5 mm; b, o, y = 0.3 mm; c = 20 μm; d, f = 15 μm; e, g–j, p, q, t, u, v, z = 10 μm; k, l, r, s, w, aa = 7 μm.
Basionym. Sphaeria rhodochlora Mont., Annls Sci. Nat., Bot., sér. 2, 1: 307. 1834.
≡ Trichosphaeria rhodochlora (Mont.) Sacc., Syll. Fung. (Abellini) 1: 454. 1882.
≡ Mattirolia rhodochlora (Mont.) Berl. (as ‘rhodoclora’), Atti Congr. Bot. Int. Genova: 574. 1892.
≡ Pleosphaeria rhodochlora (Mont.) Sacc., Syll. Fung. (Abellini) 2: 306. 1883.
= Pleosphaeria mutabilis Sacc., Syll. Fung. 2: 306. 1883.
≡ Mattirolia mutabilis (Sacc.) Checa, M.N. Blanco & G. Moreno, Mycotaxon 125: 153. 2013.
≡ Strickeria mutabilis (Sacc.) G. Winter, Rabenh. Krypt.-Fl., ed. 2, 1, 2: 288. 1885.
= Thyronectria patavina Sacc., Atti Soc. Veneto-Trentina Sci. Nat. 4: 123. 1875.
≡ Nectria patavina (Sacc.) Rossman, Mem. New York Bot. Gard. 49: 260. 1989.
≡ Valsonectria patavina (Sacc.) Cooke, Grevillea 12: 105. 1884.
= Nectria pyrrhochlora Auersw. (as ‘pyrrochlora’), in Rabenhorst, Hedwigia 8: 88. 1869.
≡ Calonectria pyrrhochlora (Auersw.) Sacc. (as ‘pyrrochlora’), Michelia 1: 251. 1878.
≡ Thyronectria pyrrhochlora (Auersw.) Sacc., Michelia 2: 325. 1881.
≡ Valsonectria pyrrochlora (Auersw.) Cooke, Grevillea 12: 105. 1884.
≡ Pleonectria pyrrhochlora (Auersw.) G. Winter, Rabenh. Krypt.-Fl., ed. 2, 1, 2, II. Abt.: Ascomyc.: Gymnoasceen: 108. 1884.
≡ Mattirolia pyrrochlora (Auersw.) Starbäck, Bih. Kongl. Svenska Vetensk.-Akad. Handl., Afd. 3 19 (no. 2): 43. 1894.
Typification. Lectotype of Sphaeria rhodochlora, here designated: FRANCE, Lyon, Chateau de Rochecardon, sub cortice alni (not Alnus, but Acer campestre or A. platanoides as determined by xylotomy), no date given, Montagne (PC0084652; MBT177536); isolectotype: FRANCE, Lyon, no date given, Montagne (K(M) 171594; as Trichosphaeria rhodochlora (Mont.) Sacc.). Lectotype of Pleosphaeria mutabilis, designated by Checa et al. (2013): FRANCE. Jura, La Bouloie, Saule marceau (Salix), soc. ?Diplodia sp., L. Quélet 406223 (UPS F-126406, as Sphaeria mutabilis). Holotype of Thyronectria patavina: ITALY, Padua, host given as Jugans regia (not supported by xylotomy), Dec. 1874, P. Saccardo (PAD). Isotype of Pleonectria pyrrhochlora: GERMANY, Arnstad, on Acer campestre, on/soc. Diplodia sp., Fleischhack, in Rabenhorst, Fungi Europaei exsiccati 1234 (WU). Epitype of Sphaeria rhodochlora, Pleosphaeria mutabilis and Thyronectria patavina, here designated: AUSTRIA, Niederösterreich, Gießhübl, on Acer campestre, on/soc. Diplodia sp., 18 Mar. 2012, H. Voglmayr (WU 31656; culture CBS 136006 = NP3; MBT177537). All mentioned type materials were studied.
Stromata immersed-erumpent from bark, at the sides usually surrounded by bark flaps, rarely superficial, stromatic tissue surrounding ascomata that are scattered or aggregated in groups of 2–40(–80) individually, sometimes uniting them into compound pulvinate stromata 0.7–5.3(–9.8) mm long (n = 29), (0.2–)0.3–0.6(–0.8) mm high (n = 25), often with rosy to light or reddish brown sides; tissue consisting of loosely or densely interwoven, (2.0–)3.0–5.5(–7.0) μm wide (n = 30) hyphae with walls to 1 μm thick, in places appearing more cellular, subhyaline to yellowish; covered by greenish yellow or light green scurf on the upper surface. Scurf amorphous, consisting of minute particles, turning brown in lactic acid. Ascomata subglobose, ellipsoid or cylindrical, (215–)300–500(–730) μm diam (n = 72) in surface view including stroma, in section (335–)435–620(–675) μm high, (175–)225–395(–505) μm diam (n = 21). Peridium orange-red, dark red, brown to nearly black when dry, (22–)30–47(–55) μm (n = 21) wide at the sides, consisting of a thin, up to 15 μm thick, inner layer of strongly compressed hyaline filiform cells and a pigmented outer layer of compressed, thick-walled cells (5.0–)5.5–12.5(–21.0) × (2.2–)3.2–5.5(–7.5) μm (n = 50), yellowish to dull orange-red in water, orange(-red) in KOH, lactic acid and 50 % glycerol, lighter and more yellow-brown at the top; cells around the ostiole small and more isodiametric; without a distinct pH-dependent colour change. Ostiolar region 65–190(–290) μm diam (n = 30), mostly obscure, usually concealed by the scurf, less commonly broad, black, smooth. Ostioles (120–)128–187(–213) μm long, at the apex (21–)48–91(–105) μm wide inside and (72–)105–180(–250) μm outside (n = 21), filled with periphyses. Periphyses narrow, 0.5–2.5 μm wide, pointed, short, 20–25 μm projecting into ostioles and slightly downwards. Apical paraphyses usually numerous, indistinct in KOH, embedded in a slime matrix when immature, descending from the top of the ascoma, richly branched and anastomosing, mostly 1.5–4.5 μm wide, free ends between ascus bases widened to 6–8 μm, sometimes becoming submoniliform, distinctly longer and wider, clearly differentiated from periphyses. Asci oblong or clavate, (92–)101–129(–137) × (16–)17–26(–31) μm (n = 30), with variable stipe and undifferentiated apex, containing 8 obliquely uniseriate or biseriate ascospores. Ascospores ellipsoid or oblong, straight or curved, (15–)18–25(–37) × (7–)9–12(–16) μm, l/w = (1.4–)1.8–2.4(–3.3) (n = 419), muriform, with (3–)5–7(–9–10) transverse and (1–)2(–3–4) longitudinal, less commonly oblique septa, hyaline and often more oblong when immature, turning yellowish to rosy or pale brownish at full maturity, smooth, sometimes budding when overmature; ascoconidia 1-celled, hyaline, oblong to mostly allantoid, (3.5–)4.2–5.5(–6.0) × (0.9–)1.1–1.4(–1.5) μm, l/w = (3.0–)3.5–4.3(–4.7) (n = 25).
Asexual morph on natural substrates — None seen.
Cultures and asexual morph — Germination of ascospores with conidia and/or hyphae; growth slow, on MEA slightly better than on CMD and PDA, on CMD at 25 °C after 10 d colony radius e.g. 27 mm, on CMD and MEA centrally inoculated plate entirely covered after 2–4 wk at 20–25 °C, colony circular, dense, colourless to yellowish or dull brownish, surface turning rosy from the centre due to conidial masses, sometimes surface after c. 1 mo covered by yellow ‘scurf’ of aerial hyphae, odour indistinct to yeast-like. Conidiation effuse; conidia formed on CMD at 25 °C within 24 h on minute pegs on hyphae in the colony or on solitary, rarely paired phialides on short, more or less erect, simple or loosely branched, narrow conidiophores. Phialides lageniform to ampulliform, (4.3–)4.5–7.7(–11.2) × (2.3–)2.5–3.5(–4.2) μm, l/w = (1.5–)1.6–2.6(–3.3) (n = 22), more or less straight, mostly inequilateral. Conidia oblong to suballantoid, 1-celled, (3.8–)4.3–7.8(–14.2) × (1.0–)1.2–2.2(–4.0) μm, l/w = (2.4–)3.2–4.3(–5.0) (n = 163), when swollen sometimes with 1–2 thin septa, smooth. Pycnidia sometimes formed in culture, e.g. in NP7 on MEA after 1 mo at RT and a further 1 mo at 15 °C. Pycnidia 0.4–0.8(–1.4) mm diam, subglobose to globose, often on a short broad stipe, solitary or in dense clusters up to 2.5 mm diam, first white, turning pale to greenish yellow, contents hyaline, with a rosy shine. Peridium pseudoparenchymatous, of cells (3.5–)4.0–7.5(–10) μm diam (n = 30) with walls to c. 1 μm thick, yellow, not changing in 3 % KOH. Conidiophores densely packed, parallel and simple or short shrub- or fan-shaped, often dichotomously branched, hyaline, filiform, 1.5–3 μm wide, cells sometimes thickened to 6 μm. Phialides solitary or in small clusters of 2–3, subulate, (9.0–)10.2–13.2(–14.3) × (1.5–)1.7–2.2(–2.5) μm, l/w = (4.4–)5.4–7.0(–7.5) (n = 30). Conidia oblong to mostly allantoid, (3.2–)4.0–5.2(–5.7) × (1.1–)1.2–1.5(–1.7) μm, l/w = (2.6–)2.8–4.0(–5.2) (n = 30), 1-celled, hyaline, smooth, eguttulate, oozing out from the pycnidial apex in mucous masses.
Habitat — On fungi, typically Diplodia spp. colonising dead corticated branches or twigs, mainly of Acer campestre, but also found on Acer opalus, Corylus avellana, Koelreuteria paniculata, Prunus tenella, Robinia pseudoacacia, Salix caprea and Ulmus minor; also recorded from Cydonia oblonga and Vitis vinifera (Seeler 1940).
Distribution — Europe (Austria, Czech Republic, France, Germany, Italy).
Other material studied (all on dead corticated twigs on the ground or attached to the trees). AUSTRIA, Burgenland, Breitenbrunn, Tenauriegel, on Ulmus minor (part a), Corylus avellana (part b) and Acer campestre (part c), on/soc. Diplodia sp., 22 Mar. 2014, H. Voglmayr & I. Greilhuber (WU 33425; culture NP8 from WU 33425a, culture NP9 from WU 33425b); Niederösterreich, Bad Fischau, on Acer campestre, on/soc. Diplodia sp., 13 Nov. 2011, H. Voglmayr (WU 31655; culture CBS 136005 = NP2); Mühlleiten, on Acer campestre, on/soc. Diplodia sp., Fusarium sp., Valsa sp. and Valsaria insitiva, 23 Mar. 2013, H. Voglmayr (WU 32149; culture NP4); Vienna, 3rd district, Botanical Garden, on Prunus tenella, on/soc. Pyrenochaeta sp. and Diplodia sp., 10 Sept. 2013, H. Voglmayr (WU 32152; culture NP7); ibid., same host, on/soc. Diplodia sp., 18 Mar. 2009, H. Voglmayr (WU 32628); ibid., on Acer campestre, on/soc. Diplodia sp., 11 Nov. 2013, W. Jaklitsch & H. Voglmayr (WU 32160); 19th district, Bellevuestraße, grid square 7763/2, on Acer campestre, on/soc. Diplodia sp. and Pyrenochaeta sp., 11 Mar. 1995, W. Jaklitsch W.J. 514 (WU 32145); between Himmelstraße and Unterer Reisenbergweg, grid square 7763/2, on Acer campestre, on/soc. Diplodia sp., Dothidotthia ramulicola, 24 Apr. 1999, W. Jaklitsch W.J. 1306 (WU 32146, BPI 746398; culture CBS 125131); 21st district, Donauturmstraße, grid square 7764/3, on Koelreuteria paniculata, on/soc. Camarosporium sp., 8 Sept. 2002, W. Jaklitsch W.J. 1935 (WU 32147); ibid., on Koelreuteria paniculata, on/soc. Diplodia sp., Camarosporium sp. and ?Pyrenochaeta pycnidia, 31 Aug. 2013, W. Jaklitsch & H. Voglmayr (WU 32150; culture NP5); ibid., on Acer campestre, on/soc. Diplodia sp., 31 Aug. 2013, H. Voglmayr & W. Jaklitsch (WU 32151; culture NP6); 22nd district, Lobau, Panozzalacke, grid square 7865/1, on Acer campestre, on/soc. Diplodia sp., 29 Oct. 2002, W. Jaklitsch W.J. 2019 (WU 32148). – FRANCE, Rougon, Gorge du Verdon, on Acer opalus, soc. Fenestella sp., 29 July 2011, H. Voglmayr (WU 31653; culture CBS 136004 = NP). – ITALY, Veneto, Galzignano, at Turri, on Robinia pseudoacacia (part a) and Acer campestre (part b), on/soc. Diplodia sp., 23 Oct. 2011, W. Jaklitsch & H. Voglmayr (WU 31654a, b; culture NP1 from WU 31654a).
Notes — As already outlined by Hirooka et al. (2012), T. rhodochlora (as Pleonectria pyrrhochlora) is similar to the closely related T. virens and T. zanthoxyli in that they have ascomata covered by bright yellowish green scurf. Additionally, ascomata of these species are embedded in stromatic tissue. Thyronectria rhodochlora differs from the other species by the width of mature ascospores averaging > 9 μm and mostly two longitudinal septa and by the absence of pycnidia. Here we report the formation of pycnidia in cultures of this species for the first time, i.e. this character is shared among the three species. Initially, ascospores of T. rhodochlora are hyaline and tend to be oblong, often curved and mostly < 10 μm wide. When fully mature, they are rosy or yellowish with a rosy tint in water, KOH and lactic acid, and this colour is characteristic for T. rhodochlora and its closest relatives, T. virens and T. zanthoxyli. No greenish colour has been seen in ascospores of any of the numerous specimens examined. Ascospores have commonly 5–7 transverse septa, but, as also shown by Saccardo (1877), a small fraction may form up to 9, rarely 10 septa. We have seen this septation consistently in fresh material of several specimens, but also in type material of P. mutabilis, T. rhodochlora and T. patavina. Thyronectria rhodochlora (formerly Pleonectria pyrrhochlora) has been thought to specifically occur on Acer campestre, but as can be seen from the list above, we have collected this species also on other trees, in association with or directly on Diplodia spp. or other fungi.
In the lecto- and isolectotype specimens of T. rhodochlora stromata are superficial on wood and partly covered by bark fibres; lower free sides of the perithecia are light to reddish brown, turning slightly more orange-red in 3 % KOH; the ostiolar area is large, convex or flattened, black, or scarcely visible and diffusely delimited due to the scurf. The apical paraphyses are numerous, the asci mostly oblong, with ascospores that are hyaline, yellowish to pale rosy, ellipsoid or oblong, partly curved, with (4–)6–7(–9) transverse and (1–)2 longitudinal septa, smooth, and not budding. Based on xylotomy and bark structure, the host is clearly a species of Acer, either A. campestre or A. platanoides.
The holotype specimen of T. patavina in PAD consists of several, partly corticated twig fragments. As already reported by Rossman et al. (1999), only little material of the described fungus remains; on two small fragments there are some scattered perithecia and one small stroma, consisting of densely aggregated, minute perithecia with yellow-green scurf. We studied a perithecial section: The perithecial wall is dull orange-red to nearly black when dry; numerous branched, 2–4 μm wide apical paraphyses are present. The asci are oblong-fusoid, with a short stipe, without a differentiated apex, each containing 8 uni- to biseriate ascospores. Ascospores are oblong to ellipsoid, also slightly inequilateral or curved, (14–)16–20(–22) × 6.5–8.5(–10.7) μm, hyaline, muriform, with 5–7(–9) transverse and 1(–3) longitudinal septa, and sometimes more irregularly septate, i.e. with some oblique instead of longitudinal septa. The host is not Juglans regia, because the twigs of the holotype do not have the characteristically chambered pith of Juglans. The overall appearance including the coarse cortical fibres below the epidermis of the bark suggests Populus sp. or Salix sp. No Thyridaria incrustans as reported by Saccardo is present, but among other fungi black pycnidia of a Diplodia sp. occur in the bark, just as usually found with T. rhodochlora. Rossman et al. (1999) studied another part of the type. They reported that the material was scant, that it contained black stromata covered by yellow-green powder, with yellowish ascomata aggregated in the stroma and included persistent paraphyses in a gelatinous matrix. The ascospores were described as hyaline, irregularly muriform, 16.5–30 × 6.8–8 μm with 5–11 and 1–3 vertical septa in clavate asci. In the material that we examined ascospores had 5–7, only rarely 9 transverse septa, as is typical for T. rhodochlora. In the original description Saccardo (1875b) gives ascospores as 25 × 9–11 μm, rarely 30 × 8 μm, with 7–9 transverse septa, which fits T. rhodochlora.
The species T. patavina was described in the same year as the genus Thyronectria, but in a different journal (see above under the synonyms). This has been cited wrongly in all available databases and papers. Only Seeler (1940) found the original, detailed species description and translated it to English. Even Saccardo (1877) himself gave the wrong page number (23 instead of 123) of his publication. The asexual morph that Saccardo (1875b) described as acervuli arranged in a valsoid manner and hyaline allantoid conidia ‘mixed with asci’ could not be detected in the residual material of the holotype of T. patavina. We, however, found ascoconidia in overmature perithecia of two recent collections of the species resembling the conidia depicted by Saccardo (1877).
The lectotype of Pleosphaeria mutabilis (UPS) includes only scant material that does not permit a re-assessment of the host plant given as Salix caprea. The perithecia are scattered and grew directly on a black host fungus with large, 2-celled, dark brown conidia or possibly ascospores that are 18–21 × 8.5–12.5 μm. These most probably represent a Diplodia sp. All morphological features of the lectotype are fully in agreement with other specimens of T. rhodochlora. Its ascospores have a yellowish colour with a pale rosy tint as shown by illustrations in Checa et al. (2013).
OTHER SPECIES OF THYRONECTRIA
Below we list species in alphabetical order, describe the three new species T. asturiensis, T. caudata and T. obscura, make 15 new combinations in Thyronectria, emend or enlarge descriptions and illustrations or epitypify several selected species that were originally described from Europe, and list some examined specimens that are available in the herbarium WU.
Thyronectria aquifolii (Fr.) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808291
Basionym. Sphaeria aquifolii Fr., Elench. Fung. 2: 82. 1828.
≡ Nectria aquifolii (Fr.) Berk., Outl. Brit. Fungol.: 393. 1860.
= Pleonectria aquifolii (Fr.) Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 106. 2012.
= Nectria inaurata Berk. & Broome, Ann. Mag. Nat. Hist., ser. II, 8: 467. 1854.
≡ Aponectria inaurata (Berk. & Broome) Sacc., Michelia 1: 296. 1878.
= Nectria aquifolii (Fr.) Berk. var. appendiculata Feltgen, Vorstud. Pilzfl. Luxemb. 3: 305. 1903.
Specimen examined. UK, Surrey, Richmond, Royal Botanic Gardens, Kew, on dead twigs of Ilex aquifolium attached to the tree, 11 Nov. 2008, H. Voglmayr (WU 30360; culture NAK = CBS 125027).
Notes — This species seems to be confined to Ilex aquifolium in Western Europe. The strain cited above is included in our phylogenetic analyses.
Thyronectria asturiensis Jaklitsch & Voglmayr, sp. nov. — MycoBank MB808293; Fig. 6
Fig. 6.
Thyronectria asturiensis WU 32124 (MA3). a–f. Stromata/ascomata (f. in 3 % KOH); g. perithecium in vertical section; h–k. asci with ascospores; l–n. peridium in section (l. ostiolar region; m. base; n. lateral; l. in lactic acid; m, n. in 3 % KOH); o. ascospores; p, q. conidiophores and phialides (20 °C, MEA, 6 d); r. conidia (20 °C, MEA, 6 d). — Scale bars: a, b, e = 0.3 mm; c, d = 0.2 mm; f = 0.5 mm; g = 100 μm; h, i, k, o, q = 10 μm; j, l, p = 15 μm; m, n = 20 μm; r = 5 μm.
Etymology. Referring to its occurrence in Asturias, Spain.
Holotype. SPAIN, Asturias, Santiago, on a black subiculum on wood of Quercus ilex, soc. Thyridaria rubronotata, 23 Mar. 2013, E. Rubio (WU 32124; culture CBS 136000 = MA3).
Stromata small, of variable outline, (0.5–)0.6–1.2(–1.4) mm long, (120–)210–420(–550) μm high (n = 20), superficial on wood or partly immersed in black hyphal subiculum of the host; stromatic tissue encasing ascomata that are scattered or aggregated in small numbers to 15, excluding the apex, individually or uniting them into compound stromata; tissue usually narrow, dull yellow to brownish, consisting of pale yellow-brown cells (5.0–)6.5–11.5(–14.0) × (3.0–)4.5–8.5(–10.5) μm (n = 20) directly around the peridium, otherwise of subhyaline to yellowish, thick-walled, 2–8(–11) μm wide hyphae, at the base interwoven with host hyphae and wood; in the upper part, except for the perithecial apex, covered by yellow, sometimes partly rosy scurf. Scurf finely granulose, turning dark brown in KOH and lactic acid, releasing some yellow pigment in KOH. Ascomata pyriform, blunt-conical to subglobose, (208–)260–400(–470) μm diam (n = 20) including stromatic tissue when dry, in section (375–)405–500(–530) μm high, (225–)250–325(–355) μm diam (n = 18), highly variable in configuration; base orange to reddish or black. Peridium (16–)18–23(–25) μm wide at the base, (13–)17–25(–30) μm at the sides (n = 18), thickened up to 85 μm around the ostiole, consisting of a narrow subhyaline inner layer of filiform cells, broader and distinct at the apex, and a pigmented outer layer of thick-walled (0.5–2 μm), compressed cells, orange to orange-red in lower regions, paler orange upward in water, only slightly more intense in 3 % KOH, slightly paler to light brown in lactic acid, paler, more longish and walls yellow incrusted in the ostiole, outwardly tending to be isodiametric. Perithecial apex (ostiolar region) (59–)62–186(–295) μm diam (n = 25) when dry, black, papillate, convex or flattened, with circular outline, centrally pierced by the minute umbilicate reddish ostiole, usually only partly covered by the yellow scurf. Ostioles (104–)107–158(–195) μm long, (39–)44–77(–108) μm wide inside at the apex, (95–)109–156(–180) μm wide outside (n = 18), with acute lanceolate periphyses partly directed downward. Apical paraphyses usually numerous, forming a reticulum, mostly 2.5–4 μm wide, descending to the bases of asci. Asci clavate or oblong, (59–)61–81(–96) × (15–)17–23(–26) μm (n = 27), with an indistinct apex and a short but variable stipe, containing 6–8 ascospores bi- to triseriate in the upper part. Ascospores oblong or inequilaterally ellipsoid, (14.0–)16.5–20.7(–22.0) × (6.3–)7.3–8.7(–9.3) μm, l/w = (1.6–)2.0–2.7(–3.0) (n = 40), straight or often curved, muriform, with (3–)5 transverse distosepta and 1 longitudinal or oblique distoseptum, first hyaline, turning green and finally dark brown when mature, with a large guttule per cell, ends broadly rounded, no sheath, not budding.
Cultures — Growth slow, on CMD colony radius < 5 mm after 7 d, 18 mm after 21 d, colony flat, lacking aerial hyphae, whitish to yellow, centre turning rosy due to conidial masses; colony radius on MEA at 20 °C, 2–3 mm after 6 d, 14 mm after 14 d; colony flat, dense, whitish to orange, margin whitish due to strands of aerial hyphae, central conidial masses mucous, hardening with time, yellow to carrot; odour unpleasant to fruity. Conidiophores emerging as fasciculate side branches on strands of parallel hyaline aerial hyphae, short, 1.8–5.2 μm wide, simple, scarcely branched, with few verticils of 2–3 branches. Phialides (4.5–)6.0–9.0(–10.8) × (2.0–)2.5–3.2(–3.5) μm, l/w = (1.8–)2.1–3.4(–4.1) (n = 40), solitary or in whorls of 2–4, lageniform, straight, curved or sigmoid, mostly inequilateral; conidia also forming on small pegs. Conidia (after 6–14 d at 20 °C on MEA and CMD) cylindrical, (3.0–)4.0–6.3(–8.2) × (1.3–)1.5–2.0(–2.7) μm, l/w = (1.9–)2.4–3.3(–4.4) (n = 120), sometimes swollen toward one end, 1-celled, smooth, straight, scarcely curved, mostly eguttulate; scar indistinct to truncate.
Habitat — On dark subicular hyphae of a fungus on dead blackened wood of Quercus ilex.
Distribution — Europe (Spain). Only known from the holotype.
Notes — In ascospore colour T. asturiensis is similar to its close relative T. roseovirens, but differs from that species in the host fungus and plant, by oblong, often curved, distoseptate ascospores that contain a single guttule per cell and the absence of pycnidia. The host is a subiculum of black hyphae, possibly belonging to the associated Thyridaria rubronotata. Mature ascospores resemble those of Thyridium vestitum. No difference was seen between conidia examined after 6 and 14 d on MEA and on CMD. They are longer and wider than those of T. roseovirens. In phylogenetic analyses (Fig. 1, 2), T. asturiensis forms a highly supported clade with T. roseovirens and T. obscura, which is remarkable, as T. obscura is morphologically quite distinct (see below).
Thyronectria aurigera (Berk. & Ravenel) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808294
Basionym. Nectria aurigera Berk. & Ravenel, Grevillea 4: 46. 1875.
≡ Calonectria aurigera (Berk. & Ravenel) Sacc., Michelia 1: 308. 1878.
≡ Pleonectria aurigera (Berk. & Ravenel) Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 110. 2012.
Thyronectria austroamericana (Speg.) Seeler, J. Arnold Arbor. 21: 405. 1940. — Fig. 7a–d
Fig. 7.
a–d: Thyronectria austroamericana WU 32664. a, b. Pycnidial aggregates on hypostromata; c. phialides; d. conidia. — e–p: Thyronectria chrysogramma. e–h. Stromata (e, f. holotype; g, h. paratype); i, n–p. ascospores; j. apical paraphyses and asci; k–m. asci (k, l. immature; k. showing oblong ascospores). — q–w. Thyronectria coryli. q, r. Perithecia with greenish yellow scurf; s. apical paraphyses and mature asci; t. ascospores and ascoconidia within asci; u. ascoconidia; v, w. asci with ascospores and ascoconidia (q: WU 32129; r: WU 30362 (NCP); s–w: WU 32127 (W.J. 1262)). — Scale bars: a, b = 1.5 mm; c, i, w = 10 μm; d, u = 5 μm; e–g, q = 0.4 mm; h, r = 0.2 mm; j = 30 μm; k–p, t, v = 15 μm; s = 75 μm.
Basionym. Pleonectria austroamericana Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 10: 22. 1880.
≡ Nectria austroamericana (Speg.) Rossman, Mem. New York Bot. Gard. 29: 257. 1989.
= Pleonectria denigrata G. Winter, Bull. Torrey Bot. Club 10: 49. 1883.
≡ Thyronectria denigrata (G. Winter) Seaver, Mycologia 1: 204. 1909.
= Pleonectria guaranitica Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 19: 44. 1885.
= Pleonectria nigropapillata Starbäck, Ark. Bot. 2: 13. 1904.
Materials examined. AUSTRIA, Vienna, 3rd district, Botanical Garden, in bark of Gymnocladus dioicus, 9 Sept. 2010, H. Voglmayr (WU 32664; culture GG). – USA, Kentucky, Lexington, on dead twigs of Gleditsia triacanthos, June 1882, W.A. Kellermann, in: Winter, L. Rabenhorstii fungi Europaei et extraeuropaei exsiccati 2948 (WU s.n., isotype of Pleonectria denigrata).
Notes — This species has been only known from North and South America, but it also occurs in Europe as asexual morph: pycnidia purplish- to dark brown, densely aggregated in large numbers, forming a strongly tubercular to cerebriform structure; conidia ellipsoid to oblong, (2.5–)2.8–3.2(–3.5) × (1.3–)1.4–1.6(–1.8) μm, l/w = (1.6–)1.8–2.2(–2.5) (n = 30).
Microscopic investigation of an isotype of Pleonectria denigrata from WU revealed the presence of numerous branched, 2–4 μm wide, apical paraphyses that are persistent in fully mature ascomata.
Thyronectria berolinensis (Sacc.) Seaver, Mycologia 1: 205. 1909
Basionym. Pleonectria berolinensis Sacc., Michelia 1: 123. 1878.
≡ Nectria berolinensis (Sacc.) Cooke, Grevillea 12: 107. 1884.
= Nectria fenestrata Berk. & M.A. Curtis, in Cooke, Grevillea 12: 81. 1884.
≡ Pleonectria fenestrata (Berk. & M.A. Curtis) Berl. & Voglino, Syll. Fung. Addit. 1–4: 216. 1886.
Materials examined. AUSTRIA, Kärnten, St. Margareten im Rosental, village area, grid square 9452/4, on Ribes rubrum, 31 July 1994, W. Jaklitsch W.J. 163 (WU 32125); ibid., 25 Oct. 1998, W. Jaklitsch W.J. 1248 (WU 32126); Vienna, 21st district, Marchfeldkanalweg, on Ribes sanguineum, 13 Apr. 2009, W. Jaklitsch (WU 30361; culture CBS 127382 = NB).
Thyronectria boothii (Hirooka, Rossman & P. Chaverri) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808295
Basionym. Pleonectria boothii Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 124. 2012.
Thyronectria caudata (Malençon) Jaklitsch & Voglmayr, comb. & stat. nov. — MycoBank MB808296; Fig. 8, 9
Fig. 8.
Thyronectria caudata, sexual morph. a–f. Perithecial aggregates (f. in 3 % KOH); g, l. perithecia and hypostroma in vertical section; h, i. peridium in section (3 % KOH; h. ostiolar region); j. hypostroma cells (3 % KOH); k. host hyphae (lactic acid); m. mature asci and apical paraphyses; n, o. asci with ascospores and ascoconidia; p, q. ascospores; r. ascoconidia. a, b, d–m, o, q, r: epitype WU 32130; c, p: holotype (MPU); n. BPI 552469. — Scale bars: a = 0.5 mm; b–f = 0.3 mm; g = 0.2 mm; h, m = 30 μm; i–k, o = 20 μm; l = 100 μm; n = 15 μm; p, q = 10 μm; r = 5 μm.
Fig. 9.
Thyronectria caudata WU 32130 (NL2), asexual morph. a, b. Pycnidia on the natural substrate (b. in association with perithecia); c. pycnidia on CMD; d–l. conidiophores and phialides (d, g. from natural host; e, f, h–l. from MEA, 20 °C, 7–9 d); m–p. conidia (m, o. from MEA, 20 °C, 7 d; n, p. from the natural host). — Scale bars: a, c = 0.2 mm; b = 0.3 mm; d, h, i, l, n = 7 μm; e, k = 15 μm; f, j = 10 μm; g, m, o, p = 5 μm.
Basionym. Thyronectria lamyi var. caudata Malençon, Bull. Trimestriel Soc. Mycol. France 95: 99. 1979.
= Thyronectria lamyi var. pakistani E. Müll. & S. Ahmad, Biologia (Lahore) 8, 2: 155. 1962.
Holotype. MOROCCO, Middle Atlas, northern slope of Bou-Ighitten, above Aguelmam N’ Sidi-Ali, elev. 2200 m, on Berberis hispanica, 30 May 1957, G. Malençon (MPU). Epitype, here designated: GREECE, Crete, path to a waste dump off the road to Omalos, on Berberis cretica, on/soc. Cucurbitaria cf. berberidis, Thyridium sp. and on inner bark, 28 Nov. 2011, W. Jaklitsch (WU 32130; culture CBS 136003 = NL2; MBT177538).
Hypostromata erumpent-superficial, crustose to pulvinate, tubercular, yellow, dark reddish to black when dry, inside yellow, 0.2–0.6 mm thick; pale yellow in water, KOH and lactic acid, pseudoparenchymatous, of isodiametric to oblong cells (4.5–)6.5–14.0(–18.5) × (3.8–)5.0–8.5(–10.0) μm (n = 30) with walls to 1 μm thick, extending as hyphae in the bark and mixed with hyphae of the host, the latter orange in lactic acid and 2.5–4.5 μm wide. Ascomata superficial on the hypostroma, typically not surrounded by bark flaps, generally aggregated in numbers of up to 42 in round or elongated clusters 1.0–2.5(–3.0) mm long (n = 12), 0.5–1.0 mm high, rarely solitary, globose to obovoid, only rarely collapsing cupulate from above, sometimes formed in 2 layers, (260–)320–467(–496) μm diam in surface view (n = 20) when dry, in section (340–)360–444(–457) μm high, (300–)350–437(–450) μm diam (n = 15), dark reddish-, purplish brown to grey-brown, deep red in 3 % KOH, laterally often covered by greenish yellow scurf of minute particles. Peridium (29–)36–67(–78) μm thick at the base, (47–)60–79(–82) μm at the sides (n = 15), up to 100 μm around the ostiole, at the base often paler and thinner and poorly delimited from the hypostroma, consisting of up to four layers laterally, the hyaline thin inner layer consisting of strongly compressed, elongate cells, the outer layers of thick-walled (1.5–2.5 μm), compressed cells (6.8–)7.0–14.8(–19.3) μm (n = 30) diam, tending to be more isodiametric outward, pigmented from outside red/yellow-orange/rosy in 3 % KOH, the pigmented part in lactic acid and 50 % glycerol brightly yellow to orange. Peridial surface with warts consisting of outer peridial cells. Ostiolar region (78–)93–169(–204) μm diam (n = 20), slightly papillate or flat-umbilicate, shiny, darker than the main part of the perithecium, red, brown to black. Ostioles (87–)89–116(–135) μm long, apically (50–)55–92(–123) μm wide inside, (99–)113–185(–235) μm outside (n = 15), periphysate. Apical paraphyses numerous, anastomosing, descending to the bases of asci, (1.5–)2–6(–8) μm wide. Asci cylindrical to clavate, (82–)104–143(–163) × (16.3–)16.5–22.3(–26.8) μm (n = 30), with 8 bi- to triseriate ascospores and mostly filled with ascoconidia when mature, with croziers and stipe of variable length; apex undifferentiated. Ascospores narrowly clavate, (21–)25–33(–39) × (4.0–)5.0–6.2(–7.2) μm, l/w = (3.5–)4.5–6.0(–7.4) (n = 103), hyaline, attenuated downward, with 5–11(–12) transverse septa and 1 longitudinal or oblique septum in 1 or few cells of the upper part, all cells budding to produce oblong, 1-celled, hyaline, mostly straight ascoconidia (2.6–)3.3–4.2(–4.7) × (0.8–)1.0–1.2(–1.4) μm, l/w = (2.5–)3.0–4.0(–5.0) (n = 127).
Asexual morph on the natural host — Pycnidia either associated with perithecia on a common hypostroma or separate, solitary or in small groups, subglobose to somewhat vertically elongated, often laterally compressed in groups, collapsed-discoid when old, dull orange-red to dark reddish brown, 0.15–0.5 mm diam, surface slightly warted. Peridium in water orange, of isodiametric cells 4–11(–13) μm diam with walls up to 1 μm thick; the interior densely clothed with numerous fascicles of shrub-like or fan-shaped, 1–2 μm wide, cylindrical conidiophores on a common, up to c. 3.5 μm wide stipe. Phialides terminal, solitary, long cylindrical, 6–12 × 1–2 μm. Conidia oblong-cylindrical, (2.8–)3.5–4.5(–5.2) × (0.9–)1.0–1.2(–1.4) μm, l/w = (2.8–)3.2–4.1(–4.8) (n = 40), hyaline, 1-celled, straight to scarcely curved, eguttulate, smooth. Sterile hyphae absent.
Cultures — On CMD conidiation sparse and mostly submerged in the agar, yellow pigment diffusing into the agar. On MEA colony radius 5 mm after 7 d at 20 °C; colony very dense, first whitish, turning yellow and finally orange by mucous, (rosy-)orange conidial masses, a yellow pigment diffusing into the agar. Conidiophores on the colony surface erect, white, (1.5–)2.0–3.5(–4.3) μm wide, comprising straight hyphae with conidia formed on short pegs, phialides scattered along the axis or brush-like to fan-shaped, consisting of a main axis with 1–3 verticils each of 2–5 steeply ascending 1- to few-celled side branches or phialides. Phialides solitary or in whorls of 2–3, lageniform to cylindrical, (5.3–)7.0–10.0(–12.2) × (1.5–)2.0–2.7(–3.2) μm, l/w = (2.1–)2.9–4.7(–6.0) (n = 46), often slightly curved, inequilateral or sigmoid, with a narrow collarette. Conidia formed holoblastically, solitary, oblong to cylindrical, (3.8–)4.5–5.7(–6.5) × (1.2–)1.4–1.8(–2.0) μm, l/w = (2.4–)2.7–3.7(–4.5) (n = 60), hyaline, 1-celled, straight or sub-allantoid, eguttulate or with few minute, often subterminal guttules, often mixed with pegs. Pycnidia observed on CMD after c. 1 mo at 15 °C subsequent to 1 wk pre-cultivation at 20 °C, forming a concentric ring on the agar surface, 0.1–0.5 mm diam, subglobose, first yellow, turning to violaceous to violaceous brown, KOH- or slightly more violaceous or purple in 3 % KOH; after 2 mo sterile; peridium a textura porrecta of short, thick-walled, pale orange, 2–4.5(–5) μm wide hyphae. Pycnidia also formed on a plug of PDA stored for 8 mo at 15 °C and placed on MEA. Conidiophores fan-shaped, phialides and conidia as described above for the effuse conidiation.
Habitat — On Cucurbitaria cf. berberidis on dead twigs of Berberis cretica, B. hispanica and B. cf. lycium.
Distribution — Mediterranean region (Southern Europe, North Africa), Asia (Pakistan).
Other materials studied. PAKISTAN, Kaghan Valley, Shogran, on Berberis cf. lycium, soc. Cucurbitaria cf. berberidis, 26 July 1956, S. Ahmad 14057 (BPI 552469, lectotype of Thyronectria lamyi var. pakistani, here designated; MBT198113); same place and host, 13 June 1967, S. Ahmad 20137 (BPI 552468); Naran, on Berberis cf. lycium, soc. Cucurbitaria cf. berberidis, 12 Aug. 1968, S. Ahmad 21198 (BPI 552470); Naran, Nathia Gali, on Berberis cf. lycium, 22 Aug. 1968, S. Ahmad 21223 (BPI 552471). – SPAIN, Andalucía, Granada, El Trevenque, above Jardín Botanico La Cortijuela, elev. c. 1700 m, on Berberis hispanica, soc. Cucurbitaria cf. berberidis, 14 May 2014, S. Tello & W. Jaklitsch (WU 33428; culture NCA1); Andalucía, Jaén, Jaén, La Pandera, N37°37’54" W3°46’34.4", elev. 1800 m, on Berberis hispanica, 12 May 2014, S. Tello, W. Jaklitsch, D. Extrada & D. Merino (WU 33429; culture NCA).
Notes — Material from Crete, Pakistan and Spain is in perfect agreement with the holotype. Characteristic of T. caudata, which is otherwise similar to T. lamyi, are the long, narrowly clavate ascospores, superficial ascomata that are usually not enclosed by bark flaps and particularly by narrow ascoconidia that have a l/w ratio of (2.5–)3.0–4.0(–5.0), similar to conidia of the asexual morph in nature and in culture, while ascoconidia of T. lamyi have a l/w ratio of (1.7–)2.1–2.6(–2.9). Thyronectria caudata is obviously drought-tolerant and in Europe confined to oromediterranean regions. The peridial colour of T. caudata is strongly pH-dependent, red to purple in KOH and bright yellow in lactic acid. The asexual morph of T. caudata on the natural host is virtually identical to that in culture, except for slightly narrower phialides and conidia. Thyronectria clavatispora, described from Ribes in North America (Hirooka et al. 2012, as Pleonectria clavatispora), has also clavate ascospores, but these are distinctly wider than those of T. caudata; in addition T. clavatispora has red, collabent ascomata that resemble those of e.g. T. berolinensis or T. coryli.
Thyronectria chrysogramma Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia 42: 245. 1890. — Fig. 7e–p
≡ Mattirolia chrysogramma (Ellis & Everh.) Sacc., Syll. Fung. (Abellini) 9: 993. 1891.
≡ Nectria chrysogramma (Ellis & Everh.) Rossman, Mem. New York Bot. Gard. 49: 259. 1989.
≡ Thyronectroidea chrysogramma (Ellis & Everh.) Seaver, Mycologia 1: 206. 1909.
Holotype. USA, Kansas, Manhattan, on Ulmus americana, Mar. 1889, Kellerman & Swingle 1421 (NY 00927545); paratype: USA, New York, Potsdam, on elm limbs (NY 00927565 = NY 1944).
Ascomata immersed and erumpent from bark, subglobose, 0.3–0.5 mm diam, scattered or aggregated, individually surrounded by yellowish stromatic tissue, sometimes immersed in soft, pulvinate, erumpent stromata to c. 1 mm diam, covered with yellow to yellow-green scurf. Peridium reddish, weakly reacting in 3 % KOH. Ostiolar area black, the interior of ostioles filled with numerous periphyses. Apical paraphyses numerous, richly branched, 1.5–5 μm wide. Asci c. 100–150 × 28–37 μm, clavate, containing 8 biseriate ascospores, apex undifferentiated. Ascospores (24–)27–33(–35.5) × (10–)12–15(–17) μm, l/w = (1.6–)2.0–2.5(–3.2) (n = 58), first hyaline and oblong, turning yellowish and finally medium to reddish brown, often with an olivaceous tinge and oblong or ellipsoid, with 4–9(–11) transverse and (1–)2–3 longitudinal, densely disposed eu- and distosepta, smooth.
Distribution — North America, on Ulmus americana.
Notes — The above description is based on a study of the holo- and paratype. They represent the same fungus, but in the paratype many perithecia are solitary and scattered. This fungus is clearly a species of the genus Thyronectria, resembling, in its distoseptate ascospores, T. asturiensis or T. roseovirens. Thyronectria chrysogramma is obviously fungicolous like other species of Thyronectria, as some of the perithecial aggregates occur directly on an effete coelomycete (?Diplodia sp.). Superficially, stromata look much like those of T. rhodochlora or T. roseovirens. From the latter it differs mostly by ascospore size and septation and the absence of the green colour of immature ascospores, although nearly mature ascospores have some olivaceous tinge. For good additional descriptions see Rossman et al. (1999) as Thyronectroidea chrysogramma and Checa et al. (2013) as Mattirolia chrysogramma in which the distoseptate ascospores are clearly illustrated.
Thyronectria clavatispora (Hirooka, Rossman & P. Chaverri) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808297
Basionym. Pleonectria clavatispora Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 128. 2012.
Thyronectria coryli (Fuckel) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808298; Fig. 7q–w
Basionym. Nectria coryli Fuckel, Fung. Rhenani Exsicc., suppl. 1, no. 1582. 1865.
≡ Chilonectria coryli (Fuckel) Ellis & Everh., N. Amer. Pyrenomyc.: 117. 1892.
≡ Creonectria coryli (Fuckel) Seaver, Mycologia 1: 186. 1909.
≡ Pleonectria coryli (Fuckel) Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 128. 2012.
= Coelosphaeria acervata P. Karst., Meddel. Soc. Fauna Fl. Fenn. 5: 56. 1879.
= Nectria coryli f. salicis Rehm, Ascomyceten Exsicc. No. 680. 1882.
Typification. Lectotype of Nectria coryli, designated by Hirooka et al. (2012): GERMANY, on twigs of Corylus avellana, Fuckel, Fungi Rhenani Exsiccati 1582 (FH!). Epitype here designated: AUSTRIA, Kärnten, St. Margareten im Rosental, Gupf, Brici, grid square 9452/2, on Corylus avellana, 25 July 2009, W. Jaklitsch & H. Voglmayr (WU 32129; culture CBS 137264 = NeCo1; MBT177539).
Ascomata erumpent-superficial, usually densely aggregated in convex groups on a hypostroma, covered by yellowish green scurf when immature, globose and light red when fresh, covered by yellow-green scurf when young, collapsed cupulate and dark red when dry, usually glabrous when mature. Hamathecium of descending, branched and anastomosing apical paraphyses forming a reticulum, present among immature and mature asci, not basally attached, easily removed from asci. Asci narrowly clavate to oblong, (58–)66–110(–129) × (9–)11–16(–18) μm (n = 14), containing 8 biseriate ascospores budding in the ascus to produce numerous ascoconidia when mature. Ascospores oblong, (8.5–)9.5–12.2(–13.0) × (2.5–)2.8–3.4(–3.7) μm, l/w = (2.7–)3.0–4.0(–4.5) (n = 35), with a central, non-constricted septum, hyaline, straight. Ascoconidia suballantoid, (2.8–)3.0–4.0(–4.5) × (1.3–)1.4–1.6(–1.7) μm, l/w = (1.9–)2.1–2.5(–2.7) (n = 30), hyaline, smooth, with a subterminal guttule at each end.
Habitat — On dead bark or twigs of deciduous trees, in Europe recorded on Corylus avellana, from own observations also on Betula pendula, Cornus sanguinea, Crataegus monogyna, Euonymus europaeus, Fraxinus ornus, Ligustrum vulgare, Pyrus communis, Viburnum lantana and V. opulus, often on blackened inner bark, on Corylus e.g. associated with Valsa spp., Otthia cf. spiraeae; in mixed piles of cut twigs apparently spreading from one plant host to others, in one occasion e.g. found on six different hosts in a single pile.
Distribution — Europe, North America.
Selected specimens examined. AUSTRIA, Kärnten, St. Margareten im Rosental, Stariwald, grid square 9452/4, on Viburnum lantana, soc. Diplodia sp., 26 Oct. 1998, W. Jaklitsch W.J. 1262 (WU 32127); ibid., on Betula pendula, 18 Mar. 2000, W. Jaklitsch (WU 32128); Oberösterreich, Schärding, St. Willibald, Aichet, on Pyrus communis, 22 May 2009, H. Voglmayr (WU30362, culture CBS 127384 = NCP).
Notes — We provide a short description and illustration of T. coryli in addition to the detailed description by Hirooka et al. (2012), because the greenish yellow scurf on perithecia has been rarely reported; in addition, we epitypify this species with material grown on its original host for which a culture and act, ITS-LSU, rpb1, rpb2, tef1 and tub sequences are available. In T. coryli the hamathecial threads between asci are clearly not basally attached, as they are easily removed in mounts from mature asci, more so than in other species we studied. In perithecia covered by greenish yellow scurf asci are usually immature. When collected late in the year, e.g. from the end of November in Austria, perithecia may occasionally be entirely yellow.
Thyronectria cucurbitula (Tode) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808299
Basionym. Sphaeria cucurbitula Tode, Fungi Mecklenb. sel. 2: 38. 1791.
≡ Pleonectria cucurbitula (Tode: Fr.) Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 132. 2012.
Materials examined. AUSTRIA, Kärnten, St. Margareten im Rosental, Stariwald, grid square 9452/4, on Pinus sylvestris, 18 Dec. 1994, W. Jaklitsch W.J. 388 (WU 32131); ibid., 25 Mar. 1995, W. Jaklitsch W.J. 541 (WU 32132); same village, Wograda, grid square 9452/3, on Pinus sylvestris, 26 May 1995, W. Jaklitsch W.J. 614 (WU 32133).
Thyronectria ilicicola (Hirooka, Rossman & P. Chaverri) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808300
Basionym. Pleonectria ilicicola Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 136. 2012.
Material examined. UK, Slough, Burnham Beeches, Buckinghamshire, on Ilex aquifolium, 15 Sept. 2004, W. Jaklitsch W.J. 2720 (WU 32134).
Thyronectria lamyi (Desm.) Seeler, J. Arnold Arbor. 21: 449. 1940. — Fig. 10a–n
Fig. 10.
a–n: Thyronectria lamyi. a–f. Perithecial aggregates (a. immature; d. with an ascoma of Cucurbitaria berberidis at the upper end; e. erect pycnidium surrounded by perithecia; f. superficial perithecia after bark removal); g, h. asci with ascospores and ascoconidia; i. apical paraphyses at ascus bases; j. ascoconidia; k, l. budding ascospores; m, n. sterile hyphae and conidia from pycnidium on natural host (a: epitype WU 32159 (NL3); b–n: WU 32141 (NL1)). — o, p: Mattirolia maclurae (holotype, LISE). o. ascus; p. ostioles in surface view. — Scale bars: a, c, e, f = 0.25 mm; b, d = 0.4 mm; g–i, m, o = 15 μm; j, n = 5 μm; k, l, p = 10 μm.
Basionym. Sphaeria lamyi Desm., Ann. Sci. Nat., Bot., sér. 2, 6: 246. 1836.
≡ Nectria lamyi (Desm.) De Not., Sfer. Ital. 1: 13. 1863.
≡ Pleonectria lamyi (Desm.) Sacc. (as ‘lamyii’), Michelia 1: 325. 1878.
Typification. Lectotype of Sphaeria lamyi, designated by Seeler (1940): FRANCE, Limoges, on dead branches of Berberis vulgaris, Desmazières, Plantes Cryptogames France No 839 (FH!). Epitype, here designated: AUSTRIA, Vienna, 3rd district, Botanical Garden, on Berberis vulgaris, on/soc. Cucurbitaria berberidis, 11 Nov. 2013, W. Jaklitsch & H. Voglmayr (WU 32159; ex-epitype culture CBS 137263 = NL3; MBT177540).
Ascomata superficial on hypostromata, usually surrounded by bark flaps, only rarely erumpent above bark, (200–)260–425(–470) μm diam in surface view (n = 20), varying in colour from orange over red to brown, covered by greenish yellow scurf or not. Ostiole periphysate. Apical paraphyses numerous, apically attached, mostly 2–5 μm wide, hanging down, branched and anastomosing, forming a coarse-meshed reticulum, with free widened ends near ascus bases, without basal attachment. Asci clavate, (94–)108–143(–160) × (17–)18.7–26.5(–30) μm, with croziers, thickened but otherwise undifferentiated apex, with 8 biseriate or obliquely uniseriate ascospores, usually packed with numerous ascoconidia when mature. Ascospores oblong or fusoid, (13.5–)18.5–25.5(–30.0) × (4.8–)5.5–7.2(–8.2) μm, l/w = (1.7–)2.8–4.3(–5.4) (n = 60), muriform, with 5–9 transverse septa and 1(–2) longitudinal septum, variable, budding in the ascus to produce hyaline, thin-walled, suballantoid ascoconidia (3.0–)3.3–4.2(–5.0) × (1.3–)1.5–1.7(–2.0) μm, l/w = (1.7–)2.1–2.6(–2.9) (n = 35).
Pycnidia on the natural host solitary or scattered on the inner bark in bark fissures, lacking a hypostroma or aggregated singly or in small numbers in direct association with perithecia on a common hypostroma, variable in shape, from discoid or lenticular and sometimes convoluted to subglobose or stipitateelongate to subcylindrical, 110–260 μm diam, orange-red, dark reddish- or purplish brown to nearly black, often disintegrating with orange cup-like bases remaining. Pycnidial interior with or without long, hyaline to pale yellowish or brownish, sterile, 2–4 μm wide, capillitium-like hyphae, densely packed, fasciculate conidiophores with cylindrical to subulate phialides and masses of oblong, straight or slightly curved, hyaline, smooth, 1-celled conidia, (3.4–)3.7–4.3(–4.6) × (1.3–)1.4–1.6 μm, l/w = (2.2–)2.4–3.0(–3.3) (n = 30).
Cultures — Colony on CMD at 25 °C usually reaching a radius of only a few mm after one month, pale rosy conidial masses spreading from the centre; on PDA at 25 °C colony covering the laterally inoculated 90 mm diam plate entirely after 20–30 d, whitish to yellow, mucous rosy-orange conidial masses spreading from the centre. Conidia hyaline, rod-shaped, long cylindrical or fusoid.
Distribution — Asia, Europe, North America.
Habitat — Common in Central Europe on Cucurbitaria berberidis on the naturally occurring Berberis vulgaris, in urban areas on many different planted species of Berberis, including B. aquifolium, B. candidula, B. gagnepainii and B. thunbergii.
Additional materials examined. AUSTRIA, Kärnten, St. Margareten im Rosental, shrubs in village area, grid square 9452/4, on Berberis vulgaris, 2 Dec. 1995, W. Jaklitsch W.J. 811 (WU 32136); Stariwald, grid square 9452/4, on Berberis vulgaris, 26 Oct. 1998, W. Jaklitsch W.J. 1264 (WU 32138); Niederösterreich, Schwarzensee, at the cross-country ski track, grid square 7962/3, on Berberis vulgaris, 25 Feb. 1996, W. Jaklitsch W.J. 825 (WU 32137); ibid., 6 Jan. 2000, W. Jaklitsch W.J. 1407 (WU 32139); Vienna, 19th district, Aslangasse, grid square 7763/2, on Berberis gagnepainii, 1 May 2001, W. Jaklitsch W.J. 1751 (WU 32140); Bellevuestraße, grid square 7763/2, on Berberis vulgaris, 25 Feb. 1995, W. Jaklitsch W.J. 505 (WU 32135); 21st district, Felix-Slavik-Straße, on Berberis thunbergii, 31 May 2009, W. Jaklitsch (WU 30363; culture CBS 127385 = NL); ibid., on Berberis candidula, 20 Feb. 2011, W. Jaklitsch (WU 32141; culture NL1). – SPAIN, Andalucia, Jaén, Camino de los Bojes, Valdepeñas de Jaén, N37°34’42.43" W3°47’56.54”, elev. 1275 m, on Berberis hispanica, 18 Dec. 2013, S. Tello JA-CUSSTA 7789 (WU 32165; culture NL4); Malaga, Sierra de las Nieves Natural Park, Parauta Pinsapar de la Escalereta, elev. 1150 m, on Berberis hispanica, 26 Dec. 2013, M. Becerra (WU 32166; culture NL5); Aragón, Huesca, Jaca, estuarine forest along the river Aragón, N42.55951 W0.59626, elev. 740 m, on Berberis hispanica ssp. seroi, 27 Dec. 2013, A. Lorenzo & J. Hernanz (WU 32169; culture NL7); Teruel, 3.5 km W from Cedrillas, N40°26’21" W0°53’43", 1502 m, on Berberis hispanica subsp. seroi, 12 Dec. 2013, R. Tena Lahoz RT13121201 (WU 32167; culture NL6).
Notes — We include here a short description and illustration of T. lamyi in addition to the detailed description by Hirooka et al. (2012), in order to facilitate comparison with T. caudata, to describe the apical paraphyses, to show some variation in the greenish yellow scurf on perithecia and pycnidia containing sterile hyphae. Sometimes apical paraphyses appear to be entrapped between ascus bases, but this may be only a consequence of mount preparation. The asexual morph in nature was found on the plant host Berberis vulgaris similar to that described by Hirooka et al. (2012), i.e. irregularly discoid to lenticular pycnidia lacking sterile internal hyphae, particularly when occurring separately from the sexual morph. Pycnidia in WU 32141 from B. candidula, especially when formed in association with perithecia on the same hypostroma, are typically subglobose to cylindrical and are darker, dark reddish brown to nearly black. Such pycnidia contain sterile hyphae. We here select a well-developed specimen for which a culture and act, ITS-LSU, rpb1, rpb2, tef1 and tub sequences are available as epitype to ensure nomenclatural stability. For delimitation from T. caudata, see under notes to that species. Both T. lamyi and T. caudata at least partly share the same plant hosts but differ in their ecology, the former being mesophilic with wide distribution in the temperate zone, whereas the latter has mostly been found in dry oromediterranean areas.
Thyronectria obscura Jaklitsch & Voglmayr, sp. nov. — MycoBank MB808301; Fig. 11
Fig. 11.
Thyronectria obscura. a–h. Stromata/Perithecia (h. in 3 % KOH); i. perithecium in vertical section; j, k. peridium in section in 3 % KOH (j. ostiolar region; k. base); l. stroma hyphae in lactic acid; m–o. asci with ascospores and ascoconidia, and apical paraphyses; p, q. ascospores; r–t. conidiophores and phialides (MEA, RT, 2d); u. ascoconidia; v, w. conidia (MEA, RT, 2 d) (a–e, h–l, n, p, r–t, v, w: WU 32142 (TT); f, g, m, o, q, u: WU 32143 (TT1)). — Scale bars: a, c, d, f = 0.2 mm; b, e, g, h = 0.3 mm; i = 50 μm; j–l = 25 μm; m–o = 15 μm; p–s, v, w = 10 μm; t, u = 5 μm.
Etymology. Referring to its dark colour.
Holotype. AUSTRIA, Vienna, 3rd district, Botanical Garden, on dead twigs of Tamarix tetrandra attached to the tree, 1 Aug. 2013, H. Voglmayr (WU 32142; culture TT = CBS 136923).
Stromata completely immersed in linear groups or erumpent from inner bark, at the sides usually surrounded by bark flaps; tissue surrounding ascomata that are scattered or aggregated in clusters of 2–21 individually or less commonly uniting them into compound pulvinate, 0.4–1.2(–1.9) mm long (n = 42) and 0.2–0.5(–0.7) mm high (n = 16) stromata; consisting of hyaline, pale yellow to pale brown, (1.5–)2.5–5.8(–9.7) μm wide (n = 31) hyphae with walls up to 1.5 μm, on its surface covered by a layer of brown cells corresponding to outer peridial cells, on the upper surface also partly covered by usually scant, sometimes well-developed, dark yellow-green or brown scurf of yellowish brown amorphous matter and brown hyphae, turning entirely black in 3 % KOH; below the ascomata continuing as a loose to compact hyphal network into the bark. Ascomata globose, subglobose to flask-shaped, (200–)225–325(–400) μm diam (n = 17) in surface view, in vertical section (270–)300–410(–420) μm high, (200–)250–310(–347) μm diam (n = 14), surrounded by stromatic tissue, becoming glabrous with age, black. Peridium (34–)40–52(–57) μm wide at the base, (36–)42–55(–60) μm at the sides (n = 14), thickened to c. 110 μm around the ostiole, consisting of a hyaline inner layer of thin-walled, strongly compressed, filiform cells and an outer layer of compressed cells (3.5–)6.5–15(–21) μm diam (n = 40), in the upper part hyaline, yellowish, pale orange to brown in 3 % KOH, lactic acid and water, tending to be larger and more pigmented out- and upward. Outer pale brown cells with incrusted, up to nearly 2 μm thick walls, becoming loose in the upper part to form a cellular layer beyond the surrounding stroma. Ostiolar region (62–)90–170(–220) μm diam (n = 21), broad, black, flat or convex, non-papillate, with shiny centre. Ostioles (85–)94–120(–129) μm long, (44–)54–84(–103) μm wide inside at the apex (n = 14), filled with up to 2.5 μm wide periphyses sometimes continuing down beyond the ostiole on the inner wall surface. Apical paraphyses numerous, 1.5–4.5(–8) μm wide, descending from the top as a reticulum, distinctly differentiated from the periphyses, branching-anastomosing, with ends widened to 8 μm between ascus bases. Asci clavate or oblong, (69–)75–99(–112) × (10.3–)11.8–16.5(–18.2) μm (n = 18), containing 8 biseriate ascospores, with short stipe and undifferentiated apex. Ascospores fusiform, oblong, vermiform or clavate, (12.5–)17.0–24.5(–28.5) × (3.5–)4.0–5.2(–6.5) μm, l/w = (2.6–)3.7–5.4(–6.9) (n = 61), hyaline, with 3–9(–10) transverse septa, with or without 1 longitudinal or oblique septum in 1–3 cells, straight or curved, smooth; all cells budding to produce cylindrical, hyaline, 1-celled, straight to slightly curved ascoconidia, (3.3–)3.7–4.7(–5.0) × (1.0–)1.3–1.7(–2.0) μm, l/w = (2.4–)2.6–3.4(–4.1) (n = 50).
Asexual morph on the natural host — None seen.
Cultures and asexual morph — Colony on CMD and MEA colourless, on CMD remaining hyaline, on MEA turning pale yellowish to rosy from the centre due to conidial masses; odour unpleasant, sourly. Conidiation effuse, formation of conidia commencing after short growth on minute pegs on hyphae in the colony or even before a distinct colony is formed, or on solitary, rarely paired phialides on short, more or less erect, simple or loosely branched conidiophores. Phialides lageniform to ampulliform, (4.3–)5.7–8.8(–11.5) × (2.5–)3.0–3.5(–4.0) μm, l/w = (1.3–)1.7–2.7(–3.4) (n = 67), straight to distinctly curved. Conidia (after 3 d on MEA at room temperature) oblong to sub-allantoid, (4.0–)5.0–8.0(–11.0) × (1.1–)1.7–3.2(–4.3) μm, l/w = (1.5–)2.3–3.2(–4.2) (n = 150).
Habitat — On dead twigs of Tamarix spp.
Distribution — Europe (Austria).
Additional material examined. AUSTRIA, Niederösterreich, Hagenbrunn, village entrance, on dead twigs of Tamarix parviflora attached to the tree, 18 Aug. 2013, W. Jaklitsch (WU 32143; culture TT1).
Notes — This species is difficult to interpret as a nectriaceous fungus at first sight, as the perithecia may be entirely black. As in other species of the genus, the apical paraphyses are distinctly differentiated from the periphyses. The former are easily removed from the asci in microscopic mounts by pressure on the cover slip. Remarkably, the close phylogenetic relationship of T. obscura to T. asturiensis and T. roseovirens is highly supported (Fig. 1, 2), despite their morphological differences.
Thyronectria okinawensis (Hirooka, Rossman & P. Chaverri) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808302
Basionym. Pleonectria okinawensis Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 146. 2012.
Thyronectria pinicola (Kirschst.) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808303
Basionym. Pleonectria pinicola Kirschst., Abh. Bot. Ver. Prov. Brandenburg 48: 59. 1906.
Material examined. AUSTRIA, Steiermark, Klöch, Steinrieglwald, 9261/2, on Pinus sylvestris, 17 Sept. 1996, W. Jaklitsch W.J. 947 (WU 32144).
Thyronectria pseudomissouriensis (Hirooka, Rossman & P. Chaverri) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808304
Basionym. Pleonectria pseudomissouriensis Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 153. 2012.
Thyronectria quercicola (Hirooka, Checa, Arenal & P. Chaverri) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808305
Basionym. Pleonectria quercicola Hirooka, Checa, Arenal & P. Chaverri, Stud. Mycol. 71: 157. 2012.
Thyronectria rosellinii (Carestia) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808306
Basionym. Nectria rosellinii Carestia, in Rabenh., Fung. Europ. Exs. No. 923. 1866.
≡ Pleonectria rosellinii (Carestia) Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 157. 2012.
Thyronectria roseovirens (Berl. & Bres.) Seeler, J. Arnold Arbor. 21: 455. 1940. — Fig. 12, 13
Fig. 13.
Thyronectria roseovirens, microscopic traits. a. Section of perithecium including natural host; b. perithecium in vertical section; c–e. peridium in section (c. ostiolar region with periphyses in water; d. lateral region in lactic acid; e. base in water); f. stroma cells in lactic acid; g–k. ascospores (g. immature, with projecting sheath; h. showing distosepta); l. apical paraphyses; m–s. asci, ascospores and apical paraphyses; t–w. conidiophores and phialides (MEA, RT, 3–4 d); x, y. conidia (MEA, RT, 3–4 d) (a–f, h: WU 32154 (MA1); g, i: WU 32158 (MA6); j, o, p, s: WU 32155 (MA2); k–n: lectotype (FH); q: WU 32156 (MA4); r: WU 32157 (MA5); t–y: WU 32153 (MA)). — Scale bars: a = 100 μm; b = 50 μm; c, e, p–s, v = 20 μm; d, h, l–o = 15 μm; f, i–k, t, x, y = 10 μm; g, u, w = 5 μm.
Basionym. Mattirolia roseovirens Berl. & Bres., Micromycet. Trident.: 55. 1889.
Typification. Lectotype of Mattirolia roseovirens, designated by Rossman et al. (1999): ITALY, Trentino, Trento, on branches of Laburnum anagyroides (or possibly L. alpinum; given as Cytisus laburnum), S. Bresadola, year not given (FH 00258826). Epitype here designated: ITALY, Trentino, Molveno, Lago di Molveno, Volta delle Assi, on Laburnum alpinum, on/soc. Cucurbitaria laburni, Valsaria sp., soc. asexual morph and setose pycnidia, 19 Oct. 2011, W. Jaklitsch & H. Voglmayr (WU 32154; ex-epitype culture CBS 135999 = MA1; MBT177541).
Stromata usually on black fungi immersed in bark, often only visible through fissures, typically surrounded by the epidermis of the plant host, less commonly superficial on wood; stromatic tissue thin, subhyaline to pale yellow, pseudoparenchymatous, of thick-walled (1–1.5 μm), rounded to angular cells, (3.8–)4.5–9.0(–16.0) μm diam (n = 30), variably containing some thick-walled, (2–)3–6(–8) μm wide (n = 35) hyphae. Stromatic tissue below ascomata often mixed with subicular hyphae of the fungal host, surrounding ascomata that are scattered or densely aggregated in groups of 2–60 individually or uniting them into compact, pulvinate, more or less tubercular stromata, 0.3–2.8(–6.5) mm long (n = 58), 0.3–0.7(–0.8) mm high (n = 34); stroma sides often rosy to light honey-brown or surrounded by rosy spots or tufts; surface covered by bright yellow scurf, sometimes with a rosy tone, often becoming dull green to black due to spore deposits; in 3 % KOH without a distinct macroscopic colour change; scurf typically present in coarse flakes, consisting of minute granules, brown in KOH, golden yellow in lactic acid. Ascomata globose to flask-shaped or conical, perithecia mostly 0.3–0.5 mm diam in surface view, in section (360–)405–515(–580) μm high, (170–)240–350(–360) μm diam (n = 20). Peridium (27–)30–43(–52) μm wide at the base and sides (n = 40), distinctly thickened around the ostiole, consisting of a thin inner layer of hyaline, elongate-filiform cells, and a thick outer pigmented layer of thick-walled, elongate, compressed, out- and upward more isodiametric cells (3.2–)4.5–11(–16) μm diam (n = 60), at the base often grading into the hypostroma, in the basal region orange in water, lactic acid and 50 % glycerol to red in 3 % KOH, in the upper region generally distinctly lighter, subhyaline, yellow to yellow-brown. Ostiolar region (78–)106–185(–235) μm diam (n = 51), flat-convex, sometimes prominent, dull ochre or yellowish grey when young, turning grey-green to black with age. Ostioles (95–)115–160(–170) μm long, apically (51–)62–89(–103) μm wide inside, (113–)131–189(–206) μm outside (n = 20), containing short, 1–2 μm wide periphyses. Apical paraphyses numerous, formed in a mucous matrix, 2–5 μm wide, branched and anastomosing. Asci oblong, clavate to subfusoid, (85–)96–129(–144) × 15–25(–36) μm (n = 30), with short stipe and undifferentiated apex, containing 8 uni- to biseriate ascospores. Ascospores ellipsoid to subglobose, (13–)15–20(–25.5) × (7.5–)9–11(–13) μm, l/w = (1.4–)1.6–2.0(–2.3) (n = 150), muriform, with 3–5(–6) transverse and 1–2 longitudinal distosepta, first hyaline, turning green and finally pale to medium brown at maturity, sometimes with a sheath projecting to c. 3.5 μm at the ends, cells eguttulate or finely multiguttulate when alive; rarely more fusiform and pale brownish without a preceding green stage, smooth.
Asexual morph on natural substrates — Either surrounding stromata partly as rosy margin, occurring separately in bark fissures or on the surface of the fungal host; first white mycelium formed, producing rosy to pale orange masses of convoluted hyaline hyphae, conidiophores to 6 μm wide and numerous conidia, or sometimes flat, compound, rosy or pale orange, 0.1–0.6 mm long pycnidia with variable outline, not changing colour in 3 % KOH; peridium pseudoparenchymatous, of pale yellowish, isodiametric to elongated cells (3.5–)6–11(–15) (n = 40) with walls to 1 μm thick; interior whitish and rosy mottled, consisting of numerous short, parallel, simple, filiform conidiophores arranged in palisades, mostly 2–4.5 μm wide. Conidia formed on short pegs and numerous phialides arranged solitarily or in whorls of 2–4. Phialides variable, lageniform to subulate or ampulliform, (4.2–)6.3–9.2(–11.2) × (2.0–)2.2–3.5(–4.2) μm, l/w = (1.4–)2.0–3.7(–5.3) (n = 40), straight or curved, sometimes constricted in the middle. Conidia oblong, narrowly ellipsoid or allantoid, (2.8–)3.5–5.0(–6.3) × (1.5–)1.7–2.2(–2.8) μm, l/w = (1.8–)1.9–2.6(–3.2) (n = 40), 1-celled, hyaline, smooth, eguttulate. Sometimes sulphur yellow mycelium or perithecia developing directly on the asexual morph.
Cultures and asexual morph — Ascospores germinating to produce conidia and hyphae, colony on CMD reaching a radius of c. 18 mm after 3 wk (strain MA3), conidia produced in masses within 24 h, colony hyaline, turning yellowish, centre rosy due to conidial masses; on PDA growth slower than on CMD, colony bright yellow with rosy conidial masses; on MEA growth usually faster than on CMD, colony yellow, centre turning rosy and eventually bright orange as conidial masses develop and extend, odour yeast-like. Culture from conidia predominantly hyphal, conidia formed after 2–3 d, colony turning yellow, crystals formed in the agar. Asexual morph of strain MA on MEA after 4–5 d at 25 °C: On CMD conidia formed on minute pegs along hyphae submerged in the agar. On MEA conidiophores formed on the agar surface, simple, acropleurogenous, sometimes densely aggregated and botryose, 2–5 μm wide, mostly asymmetric, with few-celled branches or phialides typically only on one side. Phialides mostly solitary, lateral on main axes, mixed with small pegs, or terminal on branches, then solitary or in pairs, lageniform, (4.5–)6.5–11.5(–16.5) × (2.0–)2.5–3.2(–3.5) μm, l/w = (1.8–)2.2–4.0(–5.7) (n = 43), straight, hooked or sigmoid. Conidia oblong to allantoid, sometimes ellipsoid, (3.7–)5.8–9.0(–14.5) × (1.3–)2.2–3.5(–5.2) μm, l/w = (1.9–)2.3–3.0(–3.7) (n = 86), hyaline, 1-celled, rarely with 1 or 2 non-constricted septa, smooth, eguttulate, scar indistinct.
Habitat — On species of Cucurbitaria, Diplodia and Valsaria occurring on dead corticated branches and twigs of Fabaceae (Genista, Laburnum, Ononis, Retama, Ulex).
Distribution — Southern Europe (Italy, Spain).
Additional materials examined. ITALY, Trentino, Volgaria, on Laburnum alpinum, soc. Diatrype sp., Cyphellopsis sp., 20 Oct. 2011, W. Jaklitsch & H. Voglmayr (WU 32155; culture MA2). – SPAIN, Andalucia, Granada, La Zubia, Cerro del Trevenque, N37°04’58" W3°30’26.3", elev. 1440 m, on Cucurbitaria sp. on Ononis aragonensis, 14 May 2014, S. Tello & W. Jaklitsch (WU 33431); Jaén, near N-432 exit to Castillo de Locubin, N37°31’31" W3°58’26.7", elev. 695 m, on Cucurbitaria sp. on Retama sphaerocarpa, 11 May 2014, W. Jaklitsch (WU 33430); near Villa Luenga, on Retama sphaerocarpa, on/soc. Valsaria cf. insitiva and Cucurbitaria sp., 23 Mar. 2011, W. Jaklitsch & H. Voglmayr (WU 32153; culture MA); Asturias, Santa Maria, Vega Cimera, on Genista florida, on wood, soc. asexual morph and Cucurbitaria sp., 4 June 2013, C. Lechat CLL 13027 (WU 32158; culture CBS 136002 = MA6); Soto de Los Infantes, near Viescas, on Genista florida, on ascomata and subiculum of Cucurbitaria sp., 4 June 2013, J. Linde (WU 32156; culture MA4); Villar de Vildas, on Genista florida, partly on Cucurbitaria sp., both morphs also on pycnidia of a Diplodia sp., soc. Coniochaeta sp., Valsaria sp., 6 June 2013, W. Jaklitsch & H. Voglmayr (WU 32157; culture from conidia CBS 136001 = MA5a; MA5: culture from ascospores); Navarra, Sorauren, on burnt, standing branches of Ulex europaeus, N42°52’14" W1°36’22", elev. 500 m, 11 Feb. 2014, J. Balda, comm. E. Rubio (WU 33424). – UNITED KINGDOM, England, South Hampshire, Southampton, Peartree Green, N50°54’ W1°22’, grid reference SU436115, on burnt branches of Ulex europaeus, 26 Apr. 2014, S. Rogerson, comm. P. Cannon (K(M) 191916).
Notes — Stromata of T. roseovirens are superficially similar to those of T. chrysogramma or other species, e.g., T. rhodochlora, but the scurf has a deep yellow tone. Sometimes they appear hypocrea-like (see Jaklitsch 2009, 2011) and are widely erumpent from bark. The apical paraphyses are numerous, and it is difficult to determine their points of attachment. A basal attachment could not be observed. In contrast to its close relative T. asturiensis, mature ascospores of T. roseovirens are generally medium brown and eguttulate or with many small guttules. Their distoseptation is evident in Checa et al. (2013: f. 5). These authors also pointed out that parts of the original material (see lectotype) was distributed to several other herbaria. The pycnidia in nature develop before the perithecia, which form directly on top with remnants of the asexual morph appearing as rosy margins of stromata.
Thyronectria rubicarpa (Cooke) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808307
Basionym. Nectria rubicarpa Cooke, Grevillea 7: 50. 1878.
≡ Pleonectria rubicarpa (Cooke) Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 162. 2012.
Thyronectria sinopica (Fr.) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808308
Basionym. Sphaeria sinopica Fr., Elench. Fung. 2: 81. 1828.
≡ Pleonectria sinopica (Fr.) Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 165. 2012.
Materials examined. AUSTRIA, Niederösterreich, Maierhöfen, asexual morph on Hedera helix, 20 June 2009, W. Jaklitsch & H. Voglmayr (WU 30364; culture from conidia NS = CBS 127386); Wöllersdorf, Dreistätten, Burgruine Starhemberg, on Hedera helix, 13 Mar. 2010, H. Voglmayr & I. Greilhuber (WU 32162).
Thyronectria strobi (Hirooka, Rossman & P. Chaverri) Jaklitsch & Voglmayr, comb. nov. — MycoBank MB808309
Basionym. Pleonectria strobi Hirooka, Rossman & P. Chaverri, in Hirooka et al., Stud. Mycol. 71: 169. 2012.
Thyronectria virens Harkn., in Ellis & Everhart, North Amer. Pyrenomyc.: 92. 1892. — Fig. 5m–w
≡ Pleonectria virens (Harkn.) Hirooka, Rossman & P. Chaverri, in Hirooka et al., Stud. Mycol. 71: 175. 2012.
Materials examined. FRANCE, Bourgogne, Dijon, Jardin de l’Arquebuse, on Ostrya carpinifolia, 13 Mar. 2014, A. Gardiennet AG14076 (WU 33426; culture NP10). – USA, California, Sausalito, on Rhus diversiloba, on/soc. Diplodia sp., H.W. Harkness (isolectotype BPI 631193, as Valsonectria virens).
Notes — Ascospore colour is the same as in T. rhodochlora, i.e. ranging from hyaline over yellowish to rosy. Also the host is a Diplodia sp., as a perithecium was found directly on a pycnidium in the isolectotype (Fig. 5o). French material differs from the isolectotype by larger ascospores ((19.0–)19.8–24.5(–26.7) × (7.5–)9.0–11.3(–12.0) μm (n = 20) vs (14–)16.5–20(–22) × (7.0–)7.5–8.8(–9.2) μm (n = 30)) with mostly two vertical septa approaching those of T. rhodochlora, but differ by multiguttulate cells, more oblong shape and rather indistinct septa. Fresh material from North America is thus necessary to find out, whether European material labelled T. virens (Hirooka et al. (2012)) or T. cf. virens (this work) is conspecific with the American taxon or an undescribed species. See Fig. 5m–s for an illustration of the isolectotype (BPI 631193) of T. virens and Fig. 5t–w for ascospores of WU 33426.
Thyronectria zanthoxyli (Peck) Ellis & Everh. (as ‘xanthoxyli’), N. Amer. Pyrenomyc.: 92. 1892. — Fig. 5x–aa
Basionym. Valsa xanthoxyli Peck, Ann. Rep. N.Y. State Mus. 31: 49. 1879.
≡ Fenestella xanthoxyli (Peck) Sacc., Syll. Fung. 2: 332. 1883.
≡ Nectria xanthoxyli (Peck) Rossman, Mem. New York Bot. Gard. 49: 264. 1989.
≡ Pleonectria zanthoxyli (Peck) Hirooka, Rossman & P. Chaverri, Stud. Mycol. 71: 177. 2012.
≡ Pseudovalsa xanthoxyli (Peck) Sacc., Syll. Fung. 2: 137. 1883.
Material examined. USA, New York, West Troy, on Zanthoxylum americanum, Oct. 1878, C.H. Peck (lectotype NYS 3611 and isolectotype NYS 3438 of Valsa xanthoxyli).
Note — This species is similar in many respects including ascospore colour to both T. rhodochlora and T. virens but has distinctly curved ascospores. See Fig. 5x–aa for an illustration of the type material.
Residual species in Thyronectria and Pleonectria
Some additional names in Thyronectria were dealt with by Seeler (1940), which he recognised as synonyms of other fungi. Based on the description and in the absence of a useful type specimen, he regarded T. sambucina Ellis & Everh., Bull. Torrey Bot. Club 24, 10: 458 (1897), as doubtful. Although he did not see the type specimen, he combined Pleonectria coffeicola Zimm., Zentbl. Bakt. ParasitKde, Abt. II 8: 183 (1902) in Thyronectria, a fungus that occurs on living leaves of Coffea in Java and is thus likely not a species of this genus. Seeler (1940) did not mention T. manihoticola Sousa da Câmara, Revista Agronomica 17, 2: 8 (extr.) (1929), described from Manihot in Portugal. Four additional species of Thyronectria were described later: T. hyperantarctica (D. Hawksw.) D. Hawksw. & Spooner, Kew Bull. 35, 3: 519 (1980) and T. inconspicua Döbbeler, Mitt. Bot. Staatssamml. München 14: 116 (1978) occurring on bryophytes in Argentina and Austria, respectively, and T. indica A. Pande & V.G. Rao, Geobios, New Rep. 7: 49 (1988) and T. odinae V.G. Rao & Varghese, Sydowia 32: 257 (1980) [1979] from India. No recent information about these species is available as well as for Pleonectria affinis Sacc., Bol. Soc. Brot., Coimbra, sér. 2 1: 139 (1922), which was not mentioned by Hirooka et al. (2012). Pleonectria affinis was described from an unidentified host in Africa and said to be similar to T. berolinensis.
Residual species in Mattirolia
The remaining three species placed in Mattirolia are M. maclurae M.T. Lucas & Sousa da Câmara, Agron. Lusit. 15, 2: 159 (1953), M. nivea Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 6: 292 (1898) [1899] and M. ohiensis (Ellis & Everh.) Checa, M.N. Blanco & G. Moreno, Mycotaxon 125: 155. 2013.
Examination of the holotype of M. maclurae (Portugal, Coimbra, Botanical Garden, on a twig of Maclura pomifera (as M. aurantiaca), 16 Apr. 1952, M.T. Lucas 1064 (LISE)) revealed a fungus immersed in bark, surrounded by reddish hyphae, with only the reddish cylindrical ostioles (80–110 μm diam) visible on the surface (Fig. 10p). The centrum of the ascomata consists of 1–2 μm wide trabeculae with indistinct septa, and narrowly clavate, fissitunicate asci with short stipe, measuring 103–115 × 13–15 μm, with 8 uni- to partly biseriate ascospores. The ascospores are ellipsoid-oblong, dark brown, muriform, with 5–7 transverse and 1(–2) longitudinal septa, (16–)17–20 × (7.0–)7.5–8.7(–9.2) μm, l/w = 2.1–2.5(–2.7) (n = 20) (Fig. 10o). Based on this morphology, M. maclurae may be assigned to the genus Karstenula. The fungus is accompanied by Valsaria cf. insitiva, an effete Diaporthe sp. and a Diplodia sp.
Mattirolia nivea (syn. Leucocrea nivea (Speg.) Sacc. & P. Syd. ex Lindau, Syll. Fung. (Abellini) 16: 601 (1902)) was synonymised with Balzania platensis Speg. by Rossman et al. (1999). Checa et al. (2013) included this species in Mattirolia; however, we have insufficient data to determine its placement. Likewise, as illustrated by Checa et al. (2013), perithecia of Mattirolia ohiensis lack yellow-green scurf and in the absence of molecular data its placement is unclear.
KEY TO SPECIES OF THYRONECTRIA
(adapted from the Pleonectria key in Hirooka et al. 2012; conidia given below are mature conidia in the sense of these authors)
1. Ascospores hyaline when immature, becoming distinctly pigmented, green to brown, muriform, distoseptate, not budding . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
1. Ascospores hyaline to yellowish or rosy, variously euseptate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4
2. Ascospores brown; on Ulmus americana; in North America . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. chrysogramma
2. Ascospores green to brown . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. On Fabaceae in Southern Europe; ascospores mostly ellipsoid, with finely multiguttulate cells when fresh . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. roseovirens
3. On Quercus ilex in Spain; ascospores mostly oblong, often curved, with one large guttule per cell when fresh . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. asturiensis
4. Ascospores in nature typically not budding . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
4. Ascospores in nature budding in or outside asci . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
5. Ascospores 1- to multiseptate but lacking longitudinal septa . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
5. Ascospores muriform . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
6. Ascospores (3–6–)7-septate, ellipsoid, oblong to allantoid, with broadly rounded ends, (15–)17–21(–25) × (4.5–)5.0–6.5(–7.3) μm; in culture conidia formed on pegs, 1-celled, long-cylindrical, (7.2–)8.7–11.3(–12.7) × (1.3–)1.5–2.2(–3.0) μm; on bark of dead deciduous trees, mainly Oleaceae . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. aurigera
6. Ascospores 1-septate, smooth or striate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
7. Ascospores striate, ellipsoid to fusiform, (13–)14–17(–18.5) × (4.5–)5.3–6.7(–7.3) μm; known from Argentina . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. pseudomissouriensis
7. Ascospores smooth . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8
8. Ascospores ellipsoid to fusiform, not constricted at the central septum, (9–)10–12(–13.5) × (3.3–)4.0–5.0(–5.7) μm; on Citrus, Gelsemium and Ilex. . . . . . . .T. rubicarpa
8. Ascospores ellipsoid to fusiform, slightly constricted at the central septum; on Hedera or Ilex. . . . . . . . . . . . . . . . . 9
9. On Ilex; ascospores ellipsoid, (9–)11–13.5(–15.5) × (4.0–)5.5–6.8(–7.5) μm; conidia in culture (5.5–)6.5–9.5(–12.5) × (2.0–)2.3–3.0(–3.3) μm. . . . . . . . . . . . . . . . . T. ilicicola
9. On Hedera; ascospores ellipsoid to fusiform, (8–)10.5–13(–14.5) × (3.7–)5.0–6.5(–8.0) μm; conidia in culture (5.2–)6.0–11.0(–13.5) × (1.0–)1.5–2.5(–3.0) μm . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. sinopica
10. Perithecia typically superficial on or partly immersed in a hypostroma . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
10. Perithecia surrounded by stromatic tissue . . . . . . . . . 13
11. Perithecia partly immersed in a hypostroma; ascospores subglobose to ellipsoid, (9.7–)10–12.5(–15) × (4.8–)6.0–7.5(–10.2) μm; on Fabaceae; in nature pycnidia forming tubercular to cerebriform masses; conidia ellipsoid to oblong, (1.7–)2.3–3.0(–3.5) × (1.0–)1.3–2.0(–2.5) μm. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. austroamericana
11. Perithecia superficial on a hypostroma . . . . . . . . . . . . 12
12. Ascospores of two sizes: micro-ascospores allantoid to short-cylindrical, (21–)25–30(–32.5) × (8.2–)9.5–12(–13) μm, macro-ascospores cylindrical, (37–)39–47(–49.5) × (10–)10.5–12(–13) μm; on Carya, in USA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .T. missouriensis
12. Ascospores uniform, oblong to ellipsoid, 17–25 × 6.5–8.5 μm; on Ribes. . . . . . . . . . . . . . . . . . . . . . T. berolinensis
13. Saffron to sienna stroma surrounding perithecia immersed in bark, scarcely protruding, with red-orange apex, asci long/slender; on Lonicera involucrata and Symphoricarpos; USA. . . . . . . . . . . . . . . . . . . . . . T. lonicerae
13. Perithecia immersed in yellowish stroma erumpent from bark; ascospores yellowish to rosy at maturity . . . . . . . . 14
14. Ascospores ellipsoid or oblong, (15–)18–25(–37) × (7–)9–12(–16) μm; chiefly on Acer campestre, but also other trees in Europe . . . . . . . . . . . . . . . . . . . . T. rhodochlora
14. Ascospores oblong and often curved, averaging < 9 μm wide. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
15. Ascospores (13–)16–21(–23) μm long; mean colony diameter 14 mm on PDA after 7 d at 25 °C; chiefly on Rhus, but also on other trees and shrubs . . . . . . . . . . T. virens
15. Ascospores (18–)19–24(–26.5) μm long, distinctly curved; mean colony diameter > 67 mm on PDA after 7 d at 25 °C; chiefly on Zanthoxylum . . . . . . . . . . . . . . .T. zanthoxyli
16. Ascospores not budding or budding outside asci in perithecia . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .17
16. Ascospores budding inside asci . . . . . . . . . . . . . . . . . . .18
17. Ascospores 1-septate, (8.7–)10–12.5(–13.5) × (3.7–)4.5–6.0(–6.8) μm; in culture conidia 1-celled, ellipsoid, fusiform or allantoid, (5–)7–10(–11.5) × (1.8–)2.0–2.8(–3.3) μm; on Castanopsis in Japan . . . . . . . . . . . . . . T. okinawensis
17. Ascospores muriform, mostly with 7 transversal septa and 1 longitudinal septum, 17–25 × 6.5–8.5 μm; in culture conidia swollen, ellipsoid, oblong-allantoid, (0–)1(–2)-septate, (9–)10–14(–20) × (2.2–)3.3–4.7(–5.5) μm; on Ribes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. berolinensis
18. Ascospores 1-septate. . . . . . . . . . . . . . . . . . 19
18. Ascospores multiseptate or muriform . . . . . . . . . . . . .20
19. Ascospores ellipsoid to fusiform, (8–)9–11(–13) × (3.2–)4–5.5(–6.5) μm; on Ilex aquifolium; in EuropeT. aquifolii
19. Ascospores cylindrical or fusiform, (8.3–)10–13(–15) × (2.2–)2.8–4(–5.3) μm; on Corylus avellana and other de-ciduous trees and shrubs. . . . . . . . . . . . . . . . . . T. coryli
20. Ascospores filiform, transversely multiseptate . . . . . . 21
20. Ascospores muriform . . . . . . . . . . . . . . . . . 24
21. Ascospores 8–15-septate, hyaline, (26–)31–44(–49) × (1.3–)2.3–4.0(–4.7) μm; on Quercus ilex ssp. rotundifolia . . . . . . . . . . . . . . . . .T. quercicola
21. Ascospores 8–44-septate, long-filiform, 22–75 μm long, hyaline; on conifers . . . . . . . . . . . . . . . . . 22
22. On Abies; ascomatal surface scaly; ascospores 8–31-septate, (22–)29–45(–60) × (1.5–)2.0–3.2(–4.0) μm . . . . . . . . . . . . . . . . . T. rosellinii
22. On Pinus; ascomatal surface generally scurfy . . . . . . . 23
23. On Pinus subg. Pinus; ascospores 15–39-septate, (33–)43–65(–75) × (2.3–)2.7–3.5(–3.7) μm . . . . T. cucurbitula
23. On Pinus subg. Strobus; ascospores 12–44-septate, (22–)33–52(–64) × (2.0–)2.2–3.2(–4.0) μm . . . . . . . T. strobi
24. Ascospores disarticulating; part-ascospores subglobose to ellipsoid, (7.7–)8.7–12(–13.5) × (5.0–)6.5–8.5(–9.0) μm; on Platanus occidentalis and Ulmus americana; in USA. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . T. chlorinella
24. Ascospores not disarticulating . . . . . . . . . . . . . . . . . . . . . . . .25
25. Ascospores subglobose to ellipsoid, (5.0–)5.5–7.5(–9.5) × (4.0–)4.5–6.5(–8.5) μm; on FabaceaeT. sphaerospora
25. Ascospores differently shaped . . . . . . . . . . . . . . . . . . . . . . 26
26. Ascospores clavate. . . . . . . . . . . . . . . . . . . . . .27
26. Ascospores ellipsoid, oblong to fusiform . . . . . . . . . . . . 28
27. Ascospores broadly clavate, (16–)18–23(–36.5) × (4.3–)4.8–6.2(–7.0) μm; on Ribes; in North America. . . . . . . . . . . . . . . . . . . . . . T. clavatispora
27. Ascospores narrowly clavate, (21–)25–33(–38) × (4.0–)5.0–6.2(–7.2) μm; on Berberis cretica, B. hispanica, B. cf. lycium; in Greece, Morocco, Spain and Pakistan. . . . . . . . . . . . . . . . . . . . . . T. caudata
28. Perithecia black; ascospores fusiform, oblong, vermiform or clavate; (12.5–)17.0–24.5(–28.5) × (3.5–)4.0–5.2(–6.5) μm; on Tamarix spp.; in Europe (Austria) . . . . . . T. obscura
28. Perithecia with shades of red. . . . . . . . . . . . . . . . . . . . . . . . 29
29. Ascospores fusoid or oblong, (13.5–)18.5–25.5(–30.0) × (4.8–)5.5–7.2(–8.2) μm; on Berberis spp. . . . . .T. lamyi
29. Ascospores ellipsoid, fusiform, cylindrical to vermiform, averaging < 5 μm wide; on conifers . . . . . . . . . . . . . . 30
30. On Picea; perithecial apex of three regions; ascospores with 7–25 transverse septa, cylindrical to vermiform, (15.5–)20–30(–36) × (2.8–)3.2–4.2(–4.5) μm; in culture conidia long-cylindrical to allantoid, (7.5–)9–11(–12.3) × (1.3–)1.5–2.0 μm. . . . . . . . . . . . . . . . . . . . . . . . T. boothii
30. On Abies or Pinus; perithecial apex of two regions; ascospores averaging > 4.5 μm wide. . . . . . . . . . . . . . . . . . . . . . . .31
31. On Abies; ascospores ellipsoid to fusiform with 5–9 transverse septa, (16–)20–24(–28.5) × (3–)4–5.5(–7) μm; in culture conidiophores not abundant; conidia subglobose to ellipsoid, (6.0–)6.5–7.2(–9.0) × (2.2–)2.5–3.3(–3.5) μm. . . . . . . . . . . . . . . . . . . . . . . . T. balsamea
31. On Pinus; ascospores ellipsoid, fusiform to vermiform with 5–15 transverse septa, (14–)18–28(–46.5) × (3.2–)4.3–5.3(–7) μm; in culture conidiophores abundant, conidia oblong, slightly swollen at both ends, (5.5–)7–11(–13) × (1.7–)2.0–2.7(–3.0) μm. . . . . . . . . . . . . . . . . . . . . . . . T. pinicola
DISCUSSION
Here we report a change in the phylogenetic placement of several fungi that until recently have been classified in the Thyridiaceae. We have been reluctant to accept the placement of Thyronectria and some other genera in the Thyridiaceae, because the genus Thyridium differs from nectriaceous fungi in the following fundamental traits: species of Thyridium s.str. (excluding Sinosphaeria J.Z. Yue & O.E. Erikss. and Bivonella (Sacc.) Sacc.) have a dark brown to black peridium, macro- and microscopically, that never shows a pH-dependent colour reaction, they have true paraphyses, asci that become easily detached in microscopic mounts and have sometimes a ring in the ascal apex. Also the more or less yellow entostroma surrounding the perithecia is KOH-negative. Stromatic tissues of Sinosphaeria and Bivonella yield a yellow pigment in KOH and ethanol, it is therefore questionable, whether they are synonyms of Thyridium as advocated by Eriksson & Yue (1989) and accepted by Checa et al. (2013). The yellow scurf of Thyronectria species also releases such pigment in 3 % KOH and to a lesser extent in ethanol. These morphological differences are also reflected by different phylogenetic positions, as Thyridium (Thyridiaceae) is a member of Sordariomycetidae (Spatafora et al. 2006), whereas Thyronectria is embedded within Nectriaceae (Hypocreomycetidae) (Fig. 1).
Here we also re-instate the genus Thyronectria for Pleonectria as recently monographed by Hirooka et al. (2012) on sound molecular and morphological evidence. One of the central themes that form the basis of our conclusions was the recollection of Thyronectria roseovirens in the area of its original collection site. This species is the generic type of Mattirolia, which has been regarded to possess true paraphyses and was therefore placed in the Thyridiaceae. Using molecular data, we determined that this fungus belongs to a genus of the Nectriaceae (Hypocreales). This raised the question whether the persistent hamathecial threads characterising this species are true paraphyses as interpreted earlier (Rossman et al. 1999, Checa et al. 2013) or apical paraphyses that originate at the top of the perithecium and continue down to the ascal bases. We found these threads in all other species we studied, but could not find any evidence that these threads may be attached to or originate in the subhymenium between asci, i.e. they seem to be in fact apical paraphyses. A study of immature perithecia of T. rhodochlora revealed that a hyaline apical cushion was present at the top of the perithecium, from which a 3-dimensional reticulum of anastomosing threads continues down to the base of the perithecium. In the Nectriaceae these threads are usually evanescent, but in at least a number of Thyronectria spp., and apparently also in Nectria himalayensis (Hirooka et al. 2012: f. 36D, E), they are persistent until maturation of ascospores. These apical paraphyses can be often removed from the asci by pressure on the cover slip of microscopic mounts, but sometimes this is difficult. Sometimes threads remain and this is apparently because they may become entrapped between asci near their bases (see e.g. T. lamyi, Fig. 10i). The stability of the apical paraphyses during development depends on the species, as sometimes septa may become strongly constricted and cells inflated.
As we have seen that the genus ‘Pleonectria’ contains long apical paraphyses that are clearly differentiated and much distinct from periphyses, the next question was which generic name should be applied. In this context Thyronectria clearly has priority. We had then to clarify the correct epithet of its type species. The type species of Thyronectria, T. patavina, is said to occur on Juglans regia in association with Thyridaria incrustans. After examination of thousands of branches and twigs of Juglans regia and J. nigra in several countries including Italy, also in Padua, in vain, we re-examined the type specimen of T. patavina in PAD. The equipment in the herbarium unfortunately did not allow us taking better images of the ascomata than that given in Fig. 3m. However, gross morphology as well as a section prepared in PAD, enabled us to make measurements in our lab and to produce illustrations of the apical paraphyses and asci with ascospores (see Fig. 4a, e, f, k, l). Only hyaline ascospores were present in the section, which are in accordance with those of fresh specimens and type material of ‘Pleonectria’ pyrrhochlora, but also with those of the type material of T. rhodochlora (see below).
In his original species description Saccardo (1875b) wrote that the perithecia are yellow-powdered outside, that the context is slightly reddening and that the ascospores are 25 × 9–11 μm, rarely 30 × 8 μm, first full of oil drops, later thinly and profusely 7–9-septate, muriform, hyaline. An ascospore size of 25 × 8–11 μm is given by Saccardo (1877) on his illustration of the fungus. These data fully agree with T. rhodochlora.
Subsequently we studied two parts of the original collection of Thyronectria rhodochlora and found that it is clearly conspecific with T. patavina and T. pyrrhochlora and that it was collected on a species of Acer, not Alnus as originally stated. As T. rhodochlora is older than both T. patavina and T. pyrrhochlora, it is the correct name for the type species of Thyronectria. Thyronectria rhodochlora is, however, not specific for Acer campestre as stated previously (Hirooka et al. 2012). The host of the T. patavina holotype specimen (PAD) is not Juglans, as noted above. A probable host of the type of T. patavina could be Populus sp. or Salix sp., the latter with somewhat higher probability, because ‘Mattirolia’ mutabilis, another synonym of T. rhodochlora, was collected on Salix caprea in France.
All these data given above and also the fact that we found T. rhodochlora close to the original collection area of T. patavina south from Padua in the Colli Euganei on Acer campestre and Robinia pseudoacacia, are sound and convincing evidence of conspecificity of T. patavina with T. rhodochlora. We therefore epitypify Thyronectria patavina with the same epitype specimen designated for Sphaeria rhodochlora to stabilize this connection also nomenclaturally.
Except for the muriform-spored Nectria antarctica and N. pseudotrichia and the recently added species characterised by didymo- or phragmospores, Seeler (1940) was essentially correct with his generic concept of Thyronectria and synonymies of genera, although the types of T. patavina and T. rhodochlora were not available to him. In essence, three species that he included, T. patavina, T. pyrrhochlora and T. rhodochlora, are here merged into one. At first sight it may be surprising that the Nectriaceae contain fungi that have green to distinctly brown ascospores, but already Seeler (1940) concluded that ascospore colour is insignificant on the generic level. Surprising is also that fungi that were thought to contain true paraphyses belong to a genus of the Nectriaceae. This can be explained by the difficulty to determine the origin and to find the ends of the hamathecial threads, i.e. their interpretation as true paraphyses was erroneous.
Morphologically, two groups of species are recognisable in Thyronectria: the first, which contains the majority of species, is characterised by superficial perithecia usually aggregated on a hypostroma, a strong pH-dependent colour reaction of the peridium in most species, and budding ascospores. In this group the yellow-green scurf is situated directly on the perithecial surface. It may be scant on mature perithecia, but this often varies within species e.g. depending on the host. Usually the scurf is more abundant on young perithecia, especially in those species where the perithecium collapses upon drying. Repeated drying and rehydration in nature apparently results in loss of the scurf particles because of mechanical stress. Apical paraphyses in this group are highly variable in abundance, can be generally easily removed from the asci in microscopic mounts, and they are often present as evanescent, sometimes submoniliform threads in mature perithecia. The species of this group we studied here, e.g. T. coryli, T. caudata and T. lamyi, all have such threads when asci are mature, i.e. when filled with ascoconidia (Fig. 7s, 9m, 10i).
The second group of species, contained within two highly supported clades at the base of Thyronectria (Fig. 1, 2), have scattered or aggregated perithecia, each of which is surrounded by a stroma that continues into the substrate. Here the scurf is situated on the stroma, not the perithecium. To this group, recognised by Hirooka et al. (2012) as containing T. austroamericana, T. rhodochlora (as P. pyrrhochlora), T. lonicerae, T. virens and T. zanthoxyli, we add T. asturiensis, T. obscura and T. roseovirens. Thyronectria chrysogramma belongs also here, although that species has not been sequenced. In this group the apical paraphyses are much more persistent than in the other, which is clearly shown by the fact that other workers (Rossman et al. 1999, Checa et al. 2013) interpreted them as true paraphyses. Even in this group cells of the paraphyses may sometimes become inflated with age, as seen in some perithecia in T. rhodochlora.
As reported by Hirooka et al. (2012), colour reactions to KOH may be obscured by the yellow scurf, but also by stromatic tissues encasing perithecia. Most species have some orange to red peridium a priori, which may result in a weak macroscopic colour reaction to KOH, thus there is a need to check for the colour reaction in lactic acid in which the peridium may turn bright yellow. This is best done on a slide using diluted lactic acid on dry perithecia or undiluted lactic acid after rehydration of the perithecia.
Earlier workers did not mention the fungicolous habit of the genus. That a species of a genus is not always specific for its preferred plant species or genus obviously depends on the host specificity of the respective fungal host. As we did not study all species of Thyronectria, we cannot confirm that all species are fungicolous, but it seems that the situation is similar to Trichoderma (syn. Hypocrea) in the Hypocreaceae, as usually various different fungi accompany ascomata of Thyronectria spp., but they often do not grow directly on macroscopically visible parts of fungi, which may mean that they can also attack hyphae of the host fungus.
Of the 26 species recognised by Hirooka et al. (2012) in Thyronectria (as Pleonectria) 19 are characterised by molecular data, i.e. many need recollection and sequencing, particularly those that were originally described from North America. For T. aurigera and T. virens only accessions are available in GenBank that were obtained from material collected in Europe (France). Hirooka et al. (2012) described seven new species in Thyronectria (as Pleonectria). We add three new species, provide DNA data for them and some additional species.
Acknowledgments
We thank the fungarium curators of BPI, FH, K, MPU, NY, NYS, PC, UPS and Walter Till at WU for sending and managing collections, to Rosella Marcucci (PAD) for access to the holotype specimen of T. patavina; Javier Balda, Manuel Becerra, Paul Cannon, Alain Gardiennet, Jorge Hernanz, Christian Lechat, Jesús Linde, Miguel Ribes Ripoll, Enrique Rubio, Salvador Tello and Raúl Tena Lahoz for providing fresh specimens or specimen data; Trix Merkx and Gerard Verkley (CBS) for managing our cultures, Wolfgang Dämon and Irmgard Greilhuber for insertion of specimens into WU. The financial support by the Austrian Science Fund (FWF; project P22081-B17) is gratefully acknowledged.
REFERENCES
- Carbone I, Kohn LM. 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castlebury LA, Rossman AY, Sung GH, et al. 2004. Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycological Research 108: 864–872. [DOI] [PubMed] [Google Scholar]
- Checa J, Natividad Blanco M, Moreno G. 2013. Contributions to the family Thyridiaceae. New data on Sphaeria mutabilis. Mycotaxon 125: 149–164. [Google Scholar]
- Eriksson OE, Yue JZ. 1989. An amended description and disposition of the genus Thyridium. Systema Ascomycetum 8: 9–16. [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis, program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hanlin RT. 1961. Studies in the genus Nectria. II. Morphology of N. gliocladioides. American Journal of Botany 48: 900–908. [Google Scholar]
- Hanlin RT. 1971. Morphology of Nectria haematococca. American Journal of Botany 58: 105–116. [Google Scholar]
- Hirooka Y, Rossman AY, Samuels GJ, et al. 2012. A monograph of Allantonectria, Nectria, and Pleonectria (Nectriaceae, Hypocreales, Ascomycota) and their pycnidial, sporodochial, and synnematous anamorphs. Studies in Mycology 71: 1–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM. 2009. European species of Hypocrea – Part I. Studies in Mycology 63: 1–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM. 2011. European species of Hypocrea – Part II: species with hyaline ascospores. Fungal Diversity 48: 1–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Komon M, Kubicek CP, et al. 2005. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia 97: 1365–1378. [DOI] [PubMed] [Google Scholar]
- Jaklitsch WM, Stadler M, Voglmayr H. 2012. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia 104: 925–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch WM, Voglmayr H. 2011. Nectria eustromatica sp. nov., an exceptional species with a hypocreaceous stroma. Mycologia 103: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Müller E, Ahmad S. 1962. Über einige neue oder bemerkenswerte Ascomyceten aus Pakistan. V. Biologia, Lahore 8: 151–162. [Google Scholar]
- Nylander JA, Wilgenbusch JC, Warren DL, et al. 2008. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24: 581–583. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Cigelnik E. 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. 1998. Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Riethmüller A, Voglmayr H, Göker M, et al. 2002. Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia 94: 834–849. [DOI] [PubMed] [Google Scholar]
- Rodríguez F, Oliver JF, Martín A, et al. 1990. The general stochastic model of nucleotide substitution. Journal of Theoretical Biology 142: 485–501. [DOI] [PubMed] [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, et al. 1999. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Saccardo PA. 1875a. Nova ascomycetum genera. Grevillea 4: 21–22. [Google Scholar]
- Saccardo PA. 1875b. Fungi Veneti novi vel critici. Series IV. Atti della Società Veneto-Trentina di Scienze Naturali 4: 101–141. [Google Scholar]
- Saccardo PA. 1877. Fungi Italici Autographice Delineati Fascs 1–4, Tab. 153. [Google Scholar]
- Samuels GJ, Dodd S, Lu B-S, et al. 2006 The Trichoderma koningii aggregate species. Studies in Mycology 56: 67–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeler EV. 1940. A monographic study of the genus Thyronectria. Journal of the Arnold Arboretum 21: 429–460. [Google Scholar]
- Silvestro D, Michalak I. 2012. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution 12: 335–337. [Google Scholar]
- Spatafora JW, Sung GH, Johnson D, et al. 2006. A five-gene phylogeny of Pezizomycotina. Mycologia 98: 1018–1028. [DOI] [PubMed] [Google Scholar]
- Stamatakis E. 2006a. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stamatakis E. 2006b Phylogenetic models of rate heterogeneity: a high performance computing perspective. In: Proceedings of the 20th International Parallel and Distributed Processing Symposium 2006, Rhodos, Greece. doi:10.1109/IPDPS.2006.1639535. [Google Scholar]
- Sung GH, Sung JM, Hywel-Jones NL, et al. 2007. A multigene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localised incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution 44: 1204–1223. [DOI] [PubMed] [Google Scholar]
- Swofford DL. 2002 PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods). Sunderland, Massachusetts, Sinauer Associates. [Google Scholar]
- Thiers B. 2014 Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/. [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2008. Prosthecium species with Stegonsporium anamorphs on Acer. Mycological Research 112: 885–905. [DOI] [PubMed] [Google Scholar]
- Voglmayr H, Jaklitsch WM. 2011. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae). Fungal Diversity 46: 133–170. [Google Scholar]
- Werle E, Schneider C, Renner M, et al. 1994. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research 22: 4354–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990 Amplified and direct sequencing of fungal ribosomal RNA genes for phylogenies. In: Innis MA, Gelfand DH, Sninsky JJ, et al. (eds), PCR protocols: A guide to methods and applications: 315–322 Academic Press, San Diego. [Google Scholar]
- Wiens JJ. 1998. Combining datasets with different phylogenetic histories. Systematic Biology 47: 568–581. [DOI] [PubMed] [Google Scholar]
- Zhang N, Castlebury LA, Miller AN, et al. 2006. An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98: 1076–1087. [DOI] [PubMed] [Google Scholar]