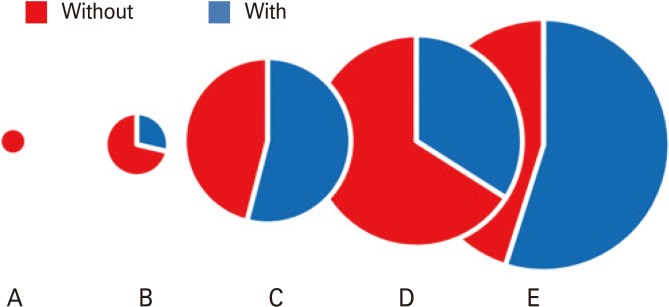
Fig. 1.
Proportion of human vaccines containing adjuvant through stages of history. Circles depict periods in vaccine development with fractional amount containing adjuvant shaded in blue, and diameter proportional to the log of number of different vaccines. A, up to 1899; B, 1900 to 1949; C, 1950 to 2012; D, 2014 U.S. licensed as listed by Food and Development Administration (FDA); E, 2014 in clinical testing as listed by HuVax (http://www.violinet.org). Note that the licensed vaccines group D contains many more multiple products for similar or overlapping indications, most of which are non-adjuvanted, while the experimental group E includes all existing and new candidate adjuvants reported in clinical testing.