Abstract
It is assumed that sheep and goats consumed the same bovine spongiform encephalopathy (BSE)-contaminated meat and bone meal that was fed to cattle and precipitated the BSE epidemic in the United Kingdom that peaked more than 20 years ago. Despite intensive surveillance for cases of BSE within the small ruminant populations of the United Kingdom and European Union, no instances of BSE have been detected in sheep, and in only two instances has BSE been discovered in goats. If BSE is present within the small ruminant populations, it may be at subclinical levels, may manifest as scrapie, or may be masked by coinfection with scrapie. To determine whether BSE is potentially circulating at low levels within the European small ruminant populations, highly sensitive assays that can specifically detect BSE, even within the presence of scrapie prion protein, are required. Here, we present a novel assay based on the specific amplification of BSE PrPSc using the serial protein misfolding cyclic amplification assay (sPMCA), which specifically amplified small amounts of ovine and caprine BSE agent which had been mixed into a range of scrapie-positive brain homogenates. We detected the BSE prion protein within a large excess of classical, atypical, and CH1641 scrapie isolates. In a blind trial, this sPMCA-based assay specifically amplified BSE PrPSc within brain mixes with 100% specificity and 97% sensitivity when BSE agent was diluted into scrapie-infected brain homogenates at 1% (vol/vol).
INTRODUCTION
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are a group of fatal neurodegenerative disorders that affect a range of mammalian species, including important food production animals and humans. The causal agent is proposed to be an abnormal conformer of a host-encoded glycoprotein known as PrPC (1), which is expressed most highly in tissues of the central nervous system (CNS). This abnormal (disease) conformer, PrPSc, is responsible for the recruitment and conversion of PrPC to further molecules of PrPSc. PrPSc has the propensity to form amyloid fibrils, and its formation is thought to be linked to the neurodegeneration of tissues within the CNS. To date, PrPSc is the only validated biomarker for these diseases. Examples of prion diseases are Creutzfeldt-Jakob disease (CJD) in humans, scrapie in sheep and goats, and chronic wasting disease (CWD) in cervids. Another prion disease, bovine spongiform encephalopathy (BSE), emerged in the United Kingdom in the mid-1980s as a result of the recycling of TSE-infected bovine slaughter offals via meat and bone meal feed additives within the ruminant feed chain. In the mid-1990s, incontrovertible biological and molecular evidence linked the consumption of BSE-contaminated material to a new human prion disease, variant CJD (2, 3), of which there have been 177 cases so far within the United Kingdom.
Sheep and goats are host to a number of prion strains, categorized as classical, atypical (4), and CH1641-like scrapie (5); each displays distinct PrPSc molecular phenotypes and pathologies. Classical scrapie is likely to be made up of many distinct strains (6). Distinguishing among the small ruminant prion strains is of considerable interest due to the possible presence of BSE and the recognition of a widespread novel prion strain, atypical scrapie, within these species (7). Strain-typing methods that can distinguish classical scrapie isolates from experimental ovine BSE and/or CH1641 isolates, including bioassays in inbred mice and immunohistochemical analysis (8), have been reported. We and others have reported rapid biochemical strain-typing tests involving the analysis of protease-resistant PrPSc. Such PrPSc types differ between classical scrapie and BSE or CH1641 in terms of their glycoform ratios and the sites of protease cleavage (9, 10). A more difficult diagnostic challenge is the distinction of ovine BSE from CH1641-like scrapie, as the two strains yield PrPScs that are indistinguishable by conventional surveillance assays (11). However, CH1641 and ovine BSE have been distinguished on the basis of a higher resistance to denaturation shown by ovine BSE isolates (12) or by multiplexed-antibody detection of protease-resistant PrPSc fragments on Western blots (13). We also recently demonstrated that ovine BSE isolates can be differentiated from classical, atypical, and CH1641 scrapie isolates by serial protein misfolding cyclic amplification (sPMCA), a methodological approach that is thought to replicate, in vitro, the prion conversion event associated with these diseases and that can be used as a sensitive assay for the detection of prions (14).
BSE prions are readily amplified under a set of defined conditions in which all scrapie isolates tested were refractory to amplification irrespective of the PRNP genotype or scrapie strain (15).
Small ruminants, which are susceptible to experimental oral challenge by the BSE agent (16), were very likely fed BSE-contaminated meat and bone meal before this material was excluded from ruminant feedstuffs. Despite intense surveillance to date, only two instances of BSE (in goats) have been detected in small ruminants (17, 18), and this very low incidence of BSE in small ruminants remains unexplained. It is possible that BSE PrPSc is not detectable by conventional PrPSc analysis either because it is present at very low levels, its molecular phenotype has evolved during interspecies transmission, and/or it is masked by scrapie during coinfections. Here, we report the development and application of a BSE-specific sPMCA to an extensive range of BSE/scrapie agent mixes which could be applied to the detection of BSE within TSE coinfections in small ruminants.
MATERIALS AND METHODS
TSE samples.
All animal procedures were performed under Home Office (United Kingdom) and local ethical review committee approval and in compliance with the Animal (Scientific Procedures) Act 1986. Scrapie samples (classical ovine and caprine, atypical ovine) from a wide geographical distribution in the United Kingdom and experimental ovine and caprine BSE-infected brain material were provided by the AHVLA biological archive. CH1641 samples from experimental challenges were provided by Nora Hunter (The Roslin Institute). In total, 54 individual scrapie isolates were used in combinations with 8 different BSE isolates. In a blind trial, we analyzed a total of 216 TSE samples (108 BSE-scrapie mixes and 108 scrapie-only samples) (Table 1). Samples were prepared as 10% (wt/vol) brain homogenates, as previously described (15).
TABLE 1.
Scrapie isolates tested in mixes with BSE
| Scrapie typea | Genotypeb | No. of isolatesc | No. of BSE isolate spikesd | No. of analysese |
|---|---|---|---|---|
| Classical | ARQ/ARQ | 4 | 6 | 8 |
| VRQ/VRQ | 6 | 6 | 12 | |
| ARQ/VRQ | 6 | 6 | 12 | |
| AHQ/AHQ | 2 | 4 | 4 | |
| AHQ/ARQ | 4 | 6 | 8 | |
| AHQ/VRQ | 3 | 5 | 6 | |
| ARH/ARH | 4 | 6 | 8 | |
| ARH/ARQ | 4 | 6 | 8 | |
| ARH/VRQ | 4 | 6 | 8 | |
| ARR/VRQ | 4 | 6 | 8 | |
| Atypical | AHQ/ARQ | 4 | 6 | 8 |
| ARR/ARR | 1 | 2 | 2 | |
| ARQ/ARQ | 1 | 2 | 2 | |
| CH1641 | AHQ/ARQ | 3 | 5 | 6 |
| Caprine scrapie | ARQ/ARQ | 4 | 2 | 8 |
| Total | 54 | 108 |
Scrapie isolates that were used in this study are listed as either classical, atypical, or CH1641 (all ovine) or caprine scrapie.
PRNP genotype of the scrapie isolate at codon positions 136, 154, and 171.
The number of scrapie isolates of each individual genotype.
The number of independent isolates of either ovine or caprine BSE that were spiked into scrapie samples of that particular genotype. Each scrapie isolate was spiked with 2 distinct BSE samples. In total, 6 ovine BSE isolates were used (4 of the ARQ/ARQ genotype, 2 of the AHQ/AHQ genotype), and 2 caprine BSE isolates were used (ARQ/ARQ genotype).
The number of individual sample mixes for each genotype that were prepared and analyzed. Total number of BSE/scrapie mixes analyzed was 108; in addition, 108 samples consisting of just the scrapie samples alone were tested.
sPMCA.
sPMCA was carried out as previously described (15). Ten percent (wt/vol) brain homogenate substrates were prepared from scrapie-free sheep from a flock with extremely high levels of biosecurity and no history of classical scrapie. After each sheep was euthanized, its whole brain was immediately removed and kept on wet ice for up to 3 h to allow transport back to the laboratory. Brain material was thoroughly cleaned in 1× phosphate-buffered saline (PBS), and the meninges, visible blood vessels, and signs of blood contamination were removed. Whole brains were then diced and flash frozen in liquid nitrogen before storage at −80°C. To prepare the sPMCA substrates, frozen brain material from animals of either the AHQ/AHQ PRNP genotype (alanine, histidine, and glutamine at codons 136, 151, and 170, respectively) or the VRQ/VRQ PRNP genotype (valine, arginine, and glutamine at codons 136, 151, and 170, respectively) were defrosted on ice and then homogenized in a blender at 10% (wt/vol) in ice-cold buffer consisting of 150 mM NaCl, 4 mM EDTA (pH 8.0), 1% (wt/vol) Triton X-100, and 1× protease inhibitor solution (Roche). The blended brain homogenate was then further homogenized by bead beating for 30 s with 1-mm glass beads. After a 10-min centrifugation at 400 × g, clarified 10% brain homogenate substrate was aliquoted and stored at −80°C until use.
Each sPMCA reaction was set up by adding the test sample at a 1-in-10 dilution into AHQ/AHQ sPMCA brain homogenate substrate to a final volume of 100 μl. Samples were sealed within 0.2-ml PCR tubes and then placed into an ultrasonicating water bath (model 4000; Misonix) at 37°C. Sonications were performed for 40 s at 200 W and repeated once every 30 min for 24 h (one sPMCA round), after which, the samples were diluted 1 in 3 with fresh substrate brain homogenate of the VRQ/VRQ genotype to a final volume of 100 μl, and the samples were subjected to another round of sPMCA. All samples were taken through a total of 5 rounds of sPMCA, using AHQ/AHQ brain substrates at rounds 1, 3, and 5 and VRQ/VRQ substrate at rounds 2 and 4.
Analysis of sPMCA products.
Reaction products were digested with proteinase K (PK) and then analyzed by Western blotting using the monoclonal antibody SHa31 as described previously (15). Briefly, samples were digested with 50 μg/ml PK in the presence of 0.045% (wt/vol) SDS for 1 h at 37°C. Samples were boiled in 1× NuPAGE SDS-PAGE sample buffer for 5 min, and then an equivalent to 2 μl of the reaction products was electrophoresed on 12% (wt/vol) polyacrylamide gels (precast NuPAGE SDS-PAGE Bis-Tris; Invitrogen). Samples were transferred to polyvinylidene difluoride (PVDF) membranes by electroblotting and then blocked in 3% skimmed milk-PBS. Blots were probed with SHa31 monoclonal antibody ascites at a dilution of 1/80,000 (Cayman Chemicals) in 0.5% (wt/vol) skimmed milk-PBS. After being washed, the blots were then probed using a secondary goat anti-mouse horseradish peroxidase (HRP) conjugate at a 1/20,000 dilution (Dako). The blots were visualized using an HRP chemiluminescent substrate (Geneflow) and a Photek imaging system. Confirmatory analysis for the single-sample replicates was carried out by the digestion of samples with PK followed by the detection of prions by Western blotting using the antibodies P4 at a 1/2,000 dilution (R-Biopharm) and SHa31 on two separate blots. Each blot was additionally loaded with a 2-μl sample of 10% brain homogenate from an ovine scrapie isolate and an ovine BSE isolate to serve as blotting controls.
Analysis of BSE/scrapie agent mixes by Western blotting.
BSE and scrapie single-isolate samples were made up in proportions from 100% BSE–0% scrapie agents in 10% increments to 0% BSE–100% scrapie agents. We chose BSE and scrapie isolates that had approximately equal amounts of PrPSc present as judged by Western blots of proteinase K-digested brain material. These mixes were analyzed on replicate Western blots using an anti-prion protein antibody, either P4 or SHa31. The blots were evaluated by densitometry of the ratio of each glycan band that made up the protease-resistant PrP triplet on the Western blots. Values for the monoglycan-versus-diglycan signal for each sample lane were plotted together with additional BSE and scrapie isolates. The BSE/scrapie agent mix samples were analyzed a total of three times for each mix on three separate Western blots.
Densitometry and determination of SHa31/P4 ratio.
For densitometry, gel images were measured with ImageJ software. The lanes on the Western blot were defined manually, and the lane pixel densities were plotted, with the areas corresponding to the band peaks defined so that the background for each lane could be subtracted. To remove subjectivity when scoring weak signals after the initial SHa31 Western blot analysis, we imposed a threshold area value of 5,800 for samples to be deemed positive in the first SHa31 screen. This threshold value was determined empirically by looking at a number of negative-control sample lanes blotted and probed in the same way as the samples under test. This figure represents the mean plus 3 standard deviations (SDs) of the SHa31 Western blot signal of 32 negative-control sPMCA samples.
The relative SHa31/P4 ratio, which is the ratio of the Western blot signals produced by amplified sPMCA products when probed with SHa31 and P4 monoclonal antibodies on two separate blots, is compared to the SHa31/P4 ratio of a control scrapie sample analyzed on the same blots (12). This relative ratio is a measure of the presence of the P4 epitope within PrPSc, present at relatively high levels in ovine scrapie isolates, but it occurs at much lower levels in ovine BSE isolates after PK digestion. The absolute SHa31/P4 ratio was determined for each test sample. This absolute ratio was then divided by the absolute ratio calculated for a scrapie control that was probed on the same blots. This resulted in a relative SHa31/P4 ratio for each sample. A cutoff value was derived by repeat analysis of ovine BSE- and scrapie-infected samples, which allowed discrimination between BSE- and scrapie-infected samples.
RESULTS
A standard methodology for the differentiation of BSE from scrapie is the analysis of the PrPSc signals obtained from dual immunoblotting of samples with antibodies directed to the core and toward the N terminus of the prion protein (9). After PK treatment, BSE PrPSc generally lacks the epitope for the more N-terminal antibody P4 but maintains reactivity to the core antibody. Scrapie PrPSc is reactive to both antibodies. Here, dual immunoblotting differentiated samples of 100% BSE agent from those of scrapie agent (Fig. 1A), but densitometry of these lanes to generate an SHa31/P4 ratio suggested that this method distinguished only the samples containing up to 80% BSE agent from scrapie agent (data not presented). In addition to dual-antibody staining, measurement of the glycoform ratios of the monoglycan and diglycan PrPSc species can also be used to discriminate prion strains (19, 20). Densitometry of the Western blot signals for the monoglycan and diglycan PrPSc species within brain mixes provided data that were plotted on a scatter plot, which demonstrated that isolates of BSE and scrapie were clearly differentiated from each other (Fig. 1B). In this format, BSE-infected samples tended to cluster with a greater proportion of diglycosylated PrP than did scrapie-infected samples. Also plotted are the mean ratios of BSE/scrapie agent mixes (mean of 3 separate analyses). These data demonstrate the insensitivity of the current discriminatory assays and show that, at best, Western blot analysis of brain samples can differentiate BSE/scrapie agent mixes containing 60% BSE agent from natural scrapie isolates.
FIG 1.
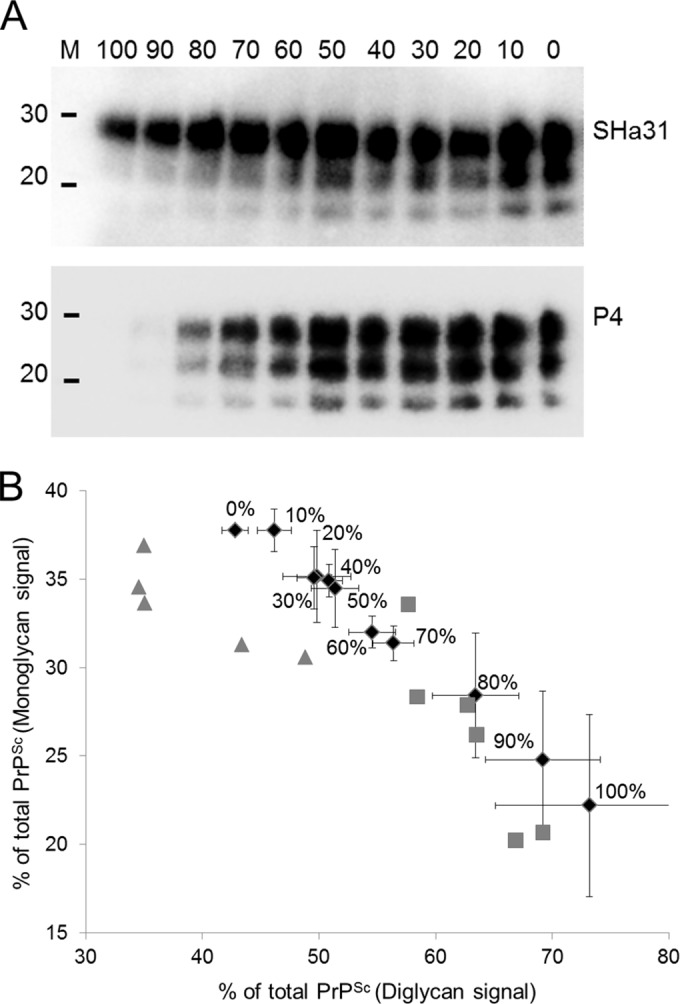
Samples of BSE- and scrapie-infected brains were PK digested, mixed in different proportions (from 100% to 0% BSE agent in 10% increments as indicated), and then Western blotted with an anti-prion antibody, either SHa31 or P4. BSE agent was immunolabeled with SHa31 but not P4 (each mixture ratio was prepared and analyzed 3 times on 3 separate Western blots). (A) Representative example of these blots. Lane M, molecular mass markers (20 and 30 kDa). (B) The relative abundance of the monoglycan and diglycan species within each of these mixed samples (SHa31 blot) was calculated by densitometry. These values are plotted as a scatter plot using the mean values of three analyses. BSE/scrapie agent mixes are plotted (black diamonds) and labeled as the percentages of BSE agent content (vol/vol), with error bars depicting the plus or minus standard error of these means. Individual experimental isolates of ovine BSE (gray squares) and isolates of classical ovine scrapie (gray triangles) are plotted as a function of their monoglycan and diglycan signals on an SHa31 Western blot. Scrapie- and BSE-infected samples clearly cluster on different sides of the plot, indicating the higher abundance of diglycosylated PrP in PK-treated BSE-infected samples than in scrapie-infected samples.
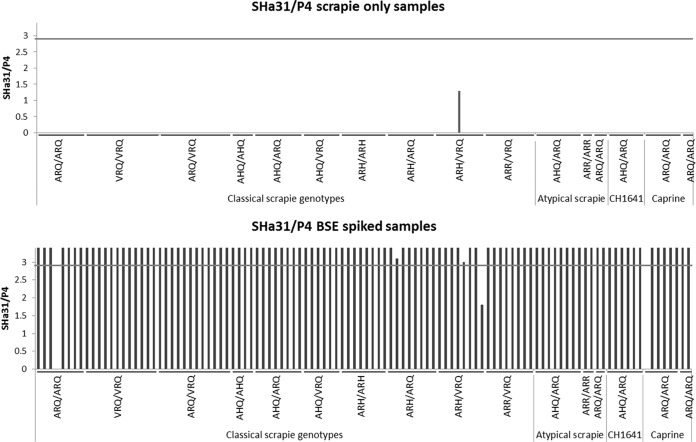
We previously detailed the selective in vitro amplification of BSE PrPSc using specific brain substrates prepared from healthy sheep (15). Here, this methodology was modified for the specific amplification of BSE PrPSc within BSE/scrapie agent mixes, a scenario which may mimic brain tissue within mixed prion infections. The sPMCA included a strategy that used ovine brain homogenate substrates from the AHQ/AHQ and VRQ/VRQ PRNP genotype substrates, which were used in alternating sPMCA rounds for a total of 5 rounds. This strategy puts greater selection for the specific BSE PrPSc amplification over the amplification of any scrapie prions present, as BSE PrPSc is amplified in both genotypes and scrapie PrPSc amplification tends to be genotype restricted. BSE-infected samples (3 isolates) were spiked into different scrapie isolates and were detected at dilutions of 1/50, 1/125, and 1/1,500, with 100%, 90%, and 80% sensitivities, respectively, after 5 rounds of sPMCA (see Tables S1 and S2 in the supplemental material). A diverse set of scrapie-infected samples from a wide geographical distribution in the United Kingdom was collected, including classical (ovine and caprine), atypical, and CH1641 scrapie isolates. BSE-infected samples came from experimentally challenged sheep and goats (Table 1). Ten percent (wt/vol) BSE- and scrapie-infected brain homogenates were prepared (10). A sample set consisting of 216 homogenates, 108 scrapie isolates only and 108 scrapie and BSE isolates mixed at 1% (vol/vol), was produced and then blinded by a third party. Samples (10 μl) were amplified in duplicate by sPMCA for a total of 5 days. The samples were digested with 50 μg/ml proteinase K and then Western blotted using the monoclonal antibody SHa31. Samples were scored positive if at least 1 reaction gave a Western blot product that was above the blotting mean background plus 3 SDs (Fig. 2A).
FIG 2.

sPMCA amplification of BSE-infected brain homogenate spiked into a 100-fold excess of scrapie agent. (A) Representative Western blots from the blind trial depicting duplicate sPMCA analyses of four separate BSE/scrapie agent mixes: lane 1, ovine BSE in ovine scrapie; lane 2, ovine BSE in atypical ovine scrapie; lane 3, classical ovine scrapie only; and lane 4, atypical ovine scrapie only. +S, PK-digested scrapie-infected brain blotting control; M, molecular mass markers (20 and 30 kDa); NEG, 4 negative-control sPMCA amplifications that contained no TSE; +BSE, positive-control amplifications that were spiked with 0.1 μl of 10% ovine BSE-infected brain only. Blots were developed with SHa31 antibody. (B) Representative examples of putative BSE sPMCA-positive samples from the blind trial probed with SHa31 (top) and P4 (bottom). Lanes 1, 3, 5, and 6, ovine BSE agent spiked into classical ovine scrapie; lanes 2 and 4, ovine BSE agent in CH1641 scrapie; lanes 7 and 8, ovine BSE agent in atypical scrapie; and lane 9, caprine BSE agent in caprine scrapie. +B, PK-digested BSE-infected brain blotting control.
A single positive replicate from the amplified samples was redigested and probed with the antibodies SHa31 and P4 on separate Western blots. Densitometric scanning of these blots determined a relative SHa31/P4 ratio. Samples with a relative SHa31/P4 ratio of ≤2.9 were defined as being of scrapie origin, and those samples with a ratio of >2.9 were defined as containing BSE agent. The value of 2.9 was derived from the densitometry analysis of 4 scrapie isolates run on 2 separate occasions and probed by SHa31 and P4. The mean SHa31/P4 ratio was 1.1, and the standard deviation in these analyses was 0.6. A threshold value was taken as this mean value plus 3 standard deviations, and this value (2.9) was used as the cutoff, below which a sample was defined as being of scrapie origin. The analysis of ovine BSE-infected brain samples on 16 separate occasions (7 different brains) gave a mean SHa31/P4 ratio of 4.5 (range, 2.9 to 7.9). In 9 of these analyses, the P4 signal was below the blot threshold; therefore, a relative ratio was not determined for these samples on these occasions. When samples were positive by SHa31 Western blotting but did not produce a measurable P4-reactive signal, they were regarded as being BSE positive.
Across all of the amplifications, 32 negative-control sPMCA samples were tested (substrate only); and they were all negative, and 31 out of 32 positive controls (amplifying 0.1 μl ovine BSE-infected brain only) were positive. The blinded sample results are summarized in Fig. 2 and 3; 108 of 108 scrapie-only samples were negative for BSE, while 105 of 108 BSE-spiked scrapie-infected samples produced a BSE-positive sPMCA product. BSE agent of goat and sheep origin was amplified under these conditions. Of the 3 BSE-spiked samples that did not amplify BSE PrPSc, 2 failed to give an amplification product, and 1 amplified but had an SHa31/P4 ratio indicative of scrapie.
FIG 3.
Summary of the relative SHa31/P4 Western blot ratios for sPMCA-amplified products. (Top) The 108 scrapie-only samples that were subjected to sPMCA. A single sample from an ARH/VRQ sheep was amplified, but its relative SHa31/P4 ratio was indicative of scrapie (below the 2.9 cutoff, shown as a gray horizontal line). The other 107 samples did not produce measurable levels of sPMCA product, as assessed by Western blotting, and a relative SHa31/P4 ratio was not recorded. (Bottom) The 108 BSE/scrapie agent mixes in which BSE-infected brain was present at 1% that of the scrapie-infected brain homogenate were analyzed. Two samples did not produce sPMCA products and are depicted on the chart without a relative SHa31/P4 ratio. One sample produced an sPMCA product but was reactive against SHa31 and P4, giving a relative SHa31/P4 ratio that was indicative of scrapie (below the SHa31/P4 2.9 relative ratio cutoff). SHa31/P4 signals for samples amplifying BSE PrPSc have been cropped to a maximum signal of 3.4, for illustrative purposes.
DISCUSSION
It is possible that within TSE coinfections, the amount of BSE agent that is present can be small and may vary in levels between tissue types, which requires very sensitive assays for detection. Strain-typing tests based on Western blot methodology are largely applicable to pure strains only (9). We demonstrate here that these methods, at best, differentiated a BSE/scrapie agent mix in a 60:40 proportion from isolates of scrapie. The detection of BSE agent that may have lower levels than this, which may be present with coinfection, requires assays that have far higher sensitivities. The sPMCA methodology described here has very high sensitivity and specificity for BSE that can be used to differentiate the BSE prion protein from classical, CH1641, and atypical scrapie prion protein, even when these different scrapie-infected brain materials were present at a 100-fold excess over BSE-infected materials. This study was carried out using a comprehensive collection of scrapie isolates within a range of PRNP genotypes. In a blind trial of 216 such samples, the BSE sPMCA test showed a specificity of 100% (all 108 negative samples tested negative) and a sensitivity of 97.2% (105 of 108 positive samples tested positive). The positive predictive value for the assay was 100% (all 105 samples that tested positive were true positives), and the negative predictive value was 97.3% (108 of the 111 samples that tested negative were true negatives). The assay should prove extremely useful alongside current screening methodology for the surveillance of small ruminants for BSE, including the analysis of scrapie-positive samples for coinfection with BSE. This test can be applied to new or historical cases of small ruminant TSE presenting with confusing pathology or molecular phenotypes (17). The methodology could also be used to inform assessments of risk to human health from animal products from experimentally mixed TSE infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by DEFRA project SE1866.
We thank the AHVLA biological archive for the provision of the BSE, classical scrapie, and atypical scrapie isolates and Nora Hunter (The Roslin Institute) for the provision of CH1641 scrapie samples.
Footnotes
Published ahead of print 20 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.01693-14.
REFERENCES
- 1.Prusiner SB. 1998. Prions. Proc. Natl. Acad. Sci. U. S. A. 95:13363–13383. 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey LJ, Lantos P. 1997. The same prion strain causes vCJD and BSE. Nature 389:448–450, 526. 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 3.Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. 1997. Transmissions to mice indicate that “new variant” CJD is caused by the BSE agent. Nature 389:498–501. 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 4.Everest SJ, Thorne L, Barnicle DA, Edwards JC, Elliott H, Jackman R, Hope J. 2006. Atypical prion protein in sheep brain collected during the British scrapie-surveillance programme. J. Gen. Virol. 87:471–477. 10.1099/vir.0.81539-0. [DOI] [PubMed] [Google Scholar]
- 5.Baron T, Bencsik A, Vulin J, Biacabe AG, Morignat E, Verchere J, Betemps DA. 2008. A C-terminal protease-resistant prion fragment distinguishes ovine “CH1641-Like” scrapie from bovine classical and L-type BSE in ovine transgenic mice. PLoS Pathog. 4(8):e1000137. 10.1371/journal.ppat.1000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seuberlich T, Botteron C, Benestad SL, Brünisholz H, Wyss R, Kihm U, Schwermer H, Friess M, Nicolier A, Heim D, Zurbriggen A. 2007. Atypical scrapie in a Swiss goat and implications for transmissible spongiform encephalopathy surveillance. J. Vet. Diagnost. Invest. 19:2–8. 10.1177/104063870701900102. [DOI] [PubMed] [Google Scholar]
- 7.Fediaevsky A, Tongue SC, Noremark M, Calavas D, Ru G, Hopp P. 2008. A descriptive study of the prevalence of atypical and classical scrapie in sheep in 20 European countries. BMC Vet. Res. 4:19. 10.1186/1746-6148-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González L, Martin S, Jeffrey M. 2003. Distinct profiles of Prp(d) immunoreactivity in the brain of scrapie- and BSE-infected sheep: implications for differential cell targeting and PrP processing. J. Gen. Virol. 84:1339–1350. 10.1099/vir.0.18800-0. [DOI] [PubMed] [Google Scholar]
- 9.Stack MJ, Chaplin MJ, Clark J. 2002. Differentiation of prion protein glycoforms from naturally occurring sheep scrapie, sheep-passaged scrapie strains (CH1641 and SSBP1), bovine spongiform encephalopathy (BSE) cases and Romney and Cheviot breed sheep experimentally inoculated with BSE using two monoclonal antibodies. Acta Neuropathol. 104:279–286 http://link.springer.com/article/10.1007%2Fs00401-002-0556-2. [DOI] [PubMed] [Google Scholar]
- 10.Owen JP, Rees HC, Maddison BC, Terry LA, Thorne L, Jackman R, Whitelam GC, Gough KC. 2007. Molecular profiling of ovine prion diseases by using thermolysin-resistant PrPSc and endogenous C2 PrP fragments. J. Virol. 81:10532–10539. 10.1128/JVI.00640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeffrey M, Gonzalez L, Chong A, Foster J, Goldmann W, Hunter N, Martin S. 2006. Ovine infection with the agents of scrapie (CH1641 isolate) and bovine spongiform encephalopathy: immunochemical similarities can be resolved by immunohistochemistry. J. Comp. Pathol. 134:17–29. 10.1016/j.jcpa.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Pirisinu L, Migliore S, Di Bari MA, Esposito E, Baron T, D'Agostino C, De Grossi L, Vaccari G, Agrimi U, Nonno R. 2011. Molecular discrimination of sheep bovine spongiform encephalopathy from scrapie. Emerg. Infect. Dis. 17:695–698. 10.3201/eid1704.101215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs JG, Sauer M, van Keulen LJ, Tang Y, Bossers A, Langeveld JP. 2011. Differentiation of ruminant transmissible spongiform encephalopathy isolate types, including bovine spongiform encephalopathy and CH1641 scrapie. J. Gen. Virol. 92:222–232. 10.1099/vir.0.026153-0. [DOI] [PubMed] [Google Scholar]
- 14.Saá P, Castilla J, Soto C. 2006. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J. Biol. Chem. 17:35245–35252. 10.1007/s12033-011-9460-0. [DOI] [PubMed] [Google Scholar]
- 15.Taema MM, Maddison BC, Thorne L, Bishop K, Owen J, Hunter N, Baker CA, Terry LA, Gough KC. 2012. Differentiating ovine BSE from CH1641 scrapie by serial protein misfolding cyclic amplification. Mol. Biotechnol. 51:233–239. 10.1007/s12033-011-9460-0. [DOI] [PubMed] [Google Scholar]
- 16.Jeffrey M, Ryder S, Martin S, Hawkins SA, Terry L, Berthelin-Baker C, Bellworthy SJ. 2001. Oral inoculation of sheep with the agent of bovine spongiform encephalopathy (BSE): 1. Onset and distribution of disease-specific PrP accumulation in brain and viscera. J. Comp. Pathol. 124:280–289. 10.1053/jcpa.2001.0465. [DOI] [PubMed] [Google Scholar]
- 17.Spiropoulos J, Lockey R, Sallis RE, Terry LA, Thorne L, Holder TM, Beck KE, Simmons MM. 2011. Isolation of prion with BSE properties from farmed goat. Emerg. Infect. Dis. 17:2253–2261. 10.3201/eid1712.110333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eloit M, Adjou K, Coulpier M, Fontaine JJ, Hamel R, Lilin T, Messiaen S, Andreoletti O, Baron T, Bencsik A, Biacabe AG, Beringue V, Laude H, Le Dur A, Vilotte JL, Comoy E, Deslys JP, Grassi J, Simon S, Lantier F, Sarradin P. 2005. BSE agent signatures in a goat. Vet. Rec. 156:523–524. [DOI] [PubMed] [Google Scholar]
- 19.Hill AF, Desbruslais M, Joiner S, Sidle KC, Gowland I, Collinge J, Doey LJ, Lantos P. 1997. The same prion strain causes vCJD and BSE. Nature 389:448–450. 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 20.Beck KE, Chaplin M, Stack M, Sallis RE, Simonini S, Lockey R, Spiropoulos J. 2010. Lesion profiling at primary isolation in RIII mice is insufficient in distinguishing BSE from classical scrapie. Brain Pathol. 20:313–322. 10.1111/j.1750-3639.2009.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.