Abstract
The 2013 WHO antiretroviral therapy (ART) guidelines recommend dried blood spots (DBS) as an alternative specimen type for viral load (VL) monitoring. We assessed the programmatic utility of screening for antiretroviral (ARV) treatment failure (TF) at 5,000 and 1,000 copies/ml using DBS and dried plasma spots (DPS) with a commonly used VL assay, the Roche Cobas Ampliprep/Cobas TaqMan V.2.0 (CAP/CTM). Plasma, DBS, and DPS were prepared from 839 whole-blood specimens collected from patients on ART for ≥6 months at three public facilities in Namibia. Using the CAP/CTM test, VL were measured in plasma, DBS, and DPS, and the results were compared using the plasma VL as the reference standard. The clinical sensitivities, specificities, and positive (PPV) and negative predictive values (NPV) of DBS at ARV TF diagnostic thresholds of 5,000 copies/ml and 1,000 copies/ml were 0.99, 0.55, 0.33, and 0.99 and 0.99, 0.26, 0.29, and 0.99, respectively, and for DPS at TF diagnostic thresholds of 5,000 copies/ml and 1,000 copies/ml, they were 0.88, 0.98, 0.92, and 0.97 and 0.91, 0.96, 0.89, and 0.97, respectively. The prevalences of TF were overestimated in DBS by 33% and 57% at these two thresholds, respectively. A high rate of false-positive results would occur if the CAP/CTM with DBS were to be used to screen for ARV TF. WHO recommendations for DBS-based VL monitoring should be specific to the VL assay version and type. Despite the better performance of DPS, the programmatic utility for TF screening may be limited by requirements for processing the whole blood at the collection site.
INTRODUCTION
In 2013, UNAIDS reported that more than 10 million people in low- and middle-income countries (LMIC) were receiving antiretroviral therapy (ART), up from 300,000 a decade ago (1). This success has accelerated efforts to reach the UNAIDS goal of treating 15 million people by 2015 (2, 3). According to the 2013 WHO ART guidelines, viral load (VL) testing is recommended as the preferred approach for monitoring ART success and diagnosing treatment failure (TF) (4). These guidelines define antiretroviral (ARV) TF as a detectable VL measured in plasma that exceeds 1,000 HIV RNA copies/ml, as opposed to 5,000 copies/ml (5).
In sub-Saharan Africa, the logistical barriers to obtaining, processing, and transporting plasma specimens to central reference laboratories for routine VL testing remain a challenge (6, 7). These logistical barriers to VL monitoring have been identified as factors contributing to lower-than-expected rates for switching ART regimens in LMIC (5). Expanding access to routine VL monitoring is a priority for ART programs in LMIC.
The standard assays used to measure VL in plasma can also be used to measure VL in dried blood spots (DBS) and dried plasma spots (DPS). Unlike plasma, DBS and DPS can be stored longer at ambient room temperatures without significant degradation of HIV RNA (8, 9). Due to the fact that storage and shipping conditions are less demanding, the use of DBS or DPS can potentially improve access to routine VL monitoring in LMIC. For this reason, DBS are recommended in the 2013 WHO guidelines as an alternative specimen type for VL monitoring.
Even though evidence suggests that the diagnostic accuracy of DBS for VL measurement at lower VL levels may be reduced (10, 11), the 2013 WHO guidelines recommend that programs using DBS for VL monitoring consider retaining a higher ARV TF diagnostic threshold of 3,000 to 5,000 copies/ml. However, additional studies suggested that the performance of DBS may be inadequate even at higher thresholds (12, 13).
The primary objective of this study was to assess the programmatic utility of routine screening for ARV TF using a common plasma VL assay, the automated Cobas Ampliprep/Cobas TaqMan V.2.0 test (CAP/CTM) (Roche Diagnostics, Ltd., Rotkreuz, Switzerland), with DBS under field laboratory conditions. We focused on the performance of the CAP/CTM with DBS at the higher (5,000 copies/ml) and lower (1,000 copies/ml) thresholds for diagnosing ARV TF that are included in the 2013 WHO guidelines. The secondary objective of this study was to assess the programmatic utility of routine screening for ARV TF using the CAP/CTM with DPS.
MATERIALS AND METHODS
Specimen collection and storage.
The 2010 Namibia ART guidelines recommend routine VL monitoring 6 months after ART initiation and targeted VL testing anytime that ARV TF is suspected by the clinician (14). VL testing is performed on plasma at the central reference laboratory in Windhoek, Namibia, using the CAP/CTM, which is an in vitro nucleic acid amplification test for the quantitation of HIV-1 RNA in human plasma. According to the WHO Prequalification of Diagnostics Programme (15), the test can quantitate HIV-1 RNA over a range of 20 to 10,000,000 copies/ml in plasma. The CAP/CTM uses an automated total nucleic acid preparation method that coextracts RNA and DNA from whole blood (16).
During this study, all specimens routinely collected for VL monitoring from patients on ART for ≥6 months at three public facilities in the Windhoek district of Namibia from August 2012 through January 2013 were eligible for inclusion. For each test, prior to plasma separation, a card with 5 spots (50 μl blood/spot) was prepared on a prepunched Whatman 903 card. After DBS preparation, and within 24 h of specimen collection, whole blood in the original EDTA tube was centrifuged to obtain plasma for the routine VL testing. If enough plasma remained after routine VL testing, one DPS test with 5 spots (50 μl blood/spot) was prepared using a Whatman 903 card. DBS and DPS were prepared with calibrated pipettes, air dried in laminar flow overnight, packed with desiccant and humidity indicator cards, and stored in individual zip-locked bags at −70°C until testing. Specimens were excluded from the study if they were collected in plasma preparation tubes or contained an insufficient amount of whole blood to generate plasma for routine VL testing and at least a single DBS card with 5 spots. The preparation and handling of plasma, DBS, and DPS were performed by certified laboratory technologists.
Plasma VL results were used to include at least 50 specimens from each of five strata: undetectable, detectable but below a low limit of detection (LOD) (<20 copies/ml), the LOD to 999 copies/ml, 1,000 to 9,999 copies/ml, and ≥10,000 copies/ml. Sampling was performed consecutively, and collection was limited to a total of 1,000 specimens in order to limit the costs of preparing unused DBS and DPS and to not prolong the study indefinitely by attempting to complete a quota for any outstanding strata. Specimens from adult and pediatric (<15-year-old) patients, and for routine and targeted monitoring, were included.
Specimen processing.
VL were measured in plasma, DBS, and DPS using the automated CAP/CTM according to the manufacturer's instructions (17). Briefly, after centrifugation, 1.1 ml of plasma from each patient was transferred into the Cobas Ampliprep specimen preparation tube and loaded onto the analyzer using the HICAP 96 protocol (Roche Diagnostics, Ltd., Rotkreuz, Switzerland). For DBS and DPS, one full prepunched circle was punched into the Cobas Ampliprep specimen preparation tube, and 1.1 ml of extraction buffer was added to the tube. The tube was then placed in a thermomixer for 10 min at 1,000 rpm and 56°C. The tubes were then loaded onto the CAP/CTM analyzer using the HISCP 96 protocol for automated processing. Testing and result interpretations were performed by certified laboratory technologists.
Statistical methods.
VL measured in plasma were analyzed descriptively with corresponding detection rates of the CAP/CTM in DBS and DPS among DBS/DPS prepared from the plasma specimens in each stratum. Bland-Altman methods were used to calculate and plot the means and standard deviations of the differences and 95% limits of agreement between paired plasma-DBS or plasma-DPS on log10-transformed data (18), and the paired t test was used to test equality of the means. Specimen pairs in which VL were undetectable or below the LOD in either half of the plasma-DBS/DPS pairs were excluded from this component of the analysis because values of VL measurement were not quantified. Using the plasma VL as a reference, the clinical sensitivities, specificities, and predictive values with 95% confidence intervals (CIs) were calculated to assess the performance of the CAP/CTM in detecting ARV TF in DBS and DPS at diagnostic thresholds of 5,000 and 1,000 copies/ml. The observed prevalences of ARV TF in plasma and DBS or DPS at the two diagnostic thresholds were also calculated overall and by reason for VL testing (i.e., routine or targeted), with differences in prevalence estimates tested for significance by McNemar's test for paired proportions. Stability was assessed by calculating the means and standard deviations of the differences and 95% limits of agreement between VL measured in DBS on day 0 and 14 and 28 days after DBS preparation. All data were analyzed using STATA V.12.
Ethical review.
Prior to implementation, the protocol was approved by the Research Committee of the Namibia Institute of Pathology and the U.S. Centers for Disease Control and Prevention. Informed patient consent is not required for receipt of routine services (including VL monitoring) performed at MOHSS facilities, and no personally identifiable information was available to the researchers. Therefore, written informed consent was not collected from any patient.
RESULTS
Among the 838 patients from whom specimens were collected and tested, 660 (79%) were from adults (≥15 years old), and 424 (51%) were from females. Of the specimens collected, 360 (43%) were for routine VL testing, and 477 (57%) were for targeted VL testing. The median plasma VL among patients who received routine and targeted testing were 125 and 245 copies/ml, respectively.
The VL was measured in 838 plasma specimens, 823 DBS, and 546 DPS. The detectable VL ranged from <20 to 2,306,918 copies/ml in plasma, <400 to 1,850,000 copies/ml in DBS, and <400 to 1,540,000 copies/ml in DPS. The VL was undetectable in 117 plasma specimens, among which the VL was detected in 15.9% of DBS and 11.8% of DPS that were prepared from those plasma specimens. The VL was detected but below the LOD in 218 plasma specimens, among which the VL was detected in 97.2% of DBS and 11.04% of DPS that were prepared from these plasma specimens (Table 1).
TABLE 1.
Detection rates of CAP/CTM among DBS or DPS prepared from plasma samples within each of five sampling strata
| Stratum and plasma VL | No. of plasma samples | No. of DBS prepared from plasma samples | No. of DBS in which VL was detecteda | DBS detection rate (CI)b | No. of DPS prepared from plasma samples | No. of DPS in which VL was detectedc | DPS detection rate (CI) |
|---|---|---|---|---|---|---|---|
| undetectable | 117 | 113 | 18 | 15.9 (9.1–22.7) | 17 | 2 | 11.8 (0.00 to 27.5) |
| <LODd | 218 | 215 | 209 | 97.2 (95.0–99.4) | 163 | 18 | 11.04 (0.00 to 15.9) |
| LOD to 999 copies/ml | 309 | 303 | 301 | 99.3 (98.4–100) | 222 | 66 | 29.73 (23.7–35.8) |
| 1,000–9,999 copies/ml | 65 | 64 | 64 | 100 | 50 | 48 | 96.0 (90.5–1.0) |
| ≥10,000 copies/ml | 129 | 128 | 128 | 100 | 94 | 93 | 98.9 (96.9–1.0) |
| Total | 838 | 823 | 720 | 546 | 227 |
Includes any DBS that was prepared from plasma specimen in stratum with detectable VL, including those at lower than the lower limit of detection (LOD), which is 400 copies/ml in DBS using the CAP/CTM.
All confidence intervals (CIs) are reported at the 95% confidence level.
Includes any DPS that was prepared from plasma specimen in stratum with detectable VL, including those at lower than the LOD, which is 400 copies/ml in DPS using the CAP/CTM.
LOD of the CAP/CTM in plasma is 20 copies/ml.
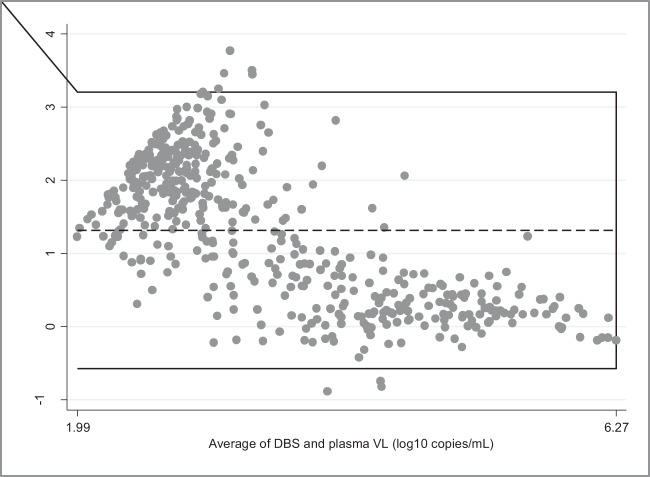
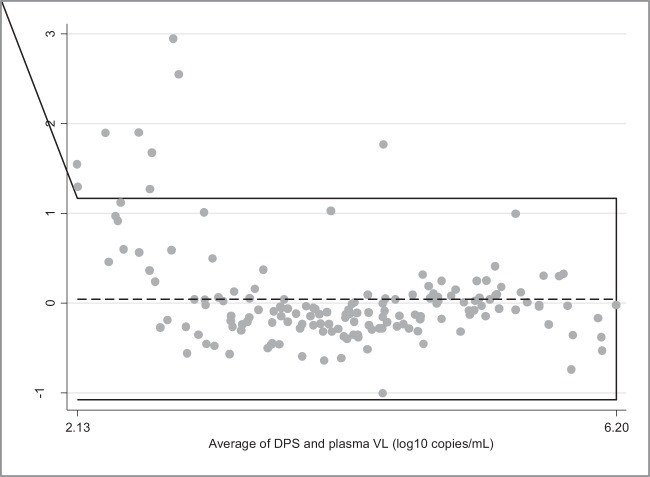
Among 485 paired plasma-DBS specimens with detectable VL measured above the LOD for plasma and DBS, the mean VL were 4.16 log10 copies/ml in DBS and 2.85 log10 copies/ml in plasma. The difference between the means (1.31 log10 copies/ml) was statistically significant (P <0.001) (Fig. 1). Among 158 paired plasma-DPS specimens with detectable VL measured above the LOD for DPS and plasma, the mean VL were 4.17 log10 copies/ml in DPS and 4.13 log10 copies/ml in plasma. The difference between the means (0.04 log10 copies/ml) was not statistically significant (P = 0.33) (Fig. 2).
FIG 1.
Bland-Altman plot showing measurement agreement between plasma and DBS VL using the CAP/CTM. The horizontal lines represent the mean difference (dotted line) and ±1.96 standard deviations (continuous lines). Limits of agreement (reference range for difference) were −0.61 to 3.24, the averages fell between 1.99 and 6.27, and the mean difference was 1.32 (95% CI, 1.23 to 1.40), with significance of difference tested by paired t test (P < 0.001). This component of the analysis included only paired plasma-DBS in which VL were quantified above the LOD in both the plasma and the DBS (n = 485).
FIG 2.
Bland-Altman plot showing measurement agreement between plasma and DPS VL using the CAP/CTM. The horizontal lines represent the mean difference (dotted line) and ±1.96 standard deviations (continuous lines). Limits of agreement (reference range for difference) were −1.10 to 1.19, the averages fell between 2.13 and 6.20, and the mean difference was 0.04 (95% CI, −0.05 to 0.13), with significance of difference tested by paired t test (P = 0.33). This analysis included only paired plasma-DPS in which VL were quantified above the LOD in both the plasma and the DPS (n = 158).
Using DBS (n = 823), the clinical sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) were 0.99, 0.55, 0.33, and 1.00, respectively, at a threshold of 5,000 copies/ml and 0.99, 0.26, 0.29, and 0.99, respectively, at a threshold of 1,000 copies/ml (Table 2). Using DPS (n = 546), the clinical sensitivities, specificities, PPV, and NPV were 0.88, 0.98, 0.92, and 0.97, respectively, at a threshold of 5,000 copies/ml and 0.91, 0.96, 0.89, and 0.97, respectively, at a threshold of 1,000 copies/ml.
TABLE 2.
Sensitivities, specificities, and predictive values of the CAP/CTM with DBS or DPS for diagnosing ARV TFa,b
| Specimen type | No. of plasma DBS or DPS pairs | Diagnostic threshold, copies/ml | Sensitivity (CI) | Specificity (CI) | PPV (CI) | NPV (CI) |
|---|---|---|---|---|---|---|
| DBS | 823 | >5,000 | 0.99 (0.96–1.00) | 0.55 (0.51–0.59) | 0.33 (0.29–0.37) | 1.00 (0.98–1.00) |
| >1,000 | 0.99 (0.97–1.00) | 0.26 (0.22–0.29) | 0.29 (0.26–0.33) | 0.99 (0.96–1.00) | ||
| DPS | 546 | >5,000 | 0.88 (0.81–0.94) | 0.98 (0.96–0.99) | 0.92 (0.85–0.97) | 0.97 (0.95–0.98) |
| >1,000 | 0.91 (0.85–0.95) | 0.96 (0.94–0.98) | 0.89 (0.83–0.94) | 0.97 (0.94–0.98) |
Plasma VL is the reference standard for sensitivity, specificity, and predictive values of VL in DBS or DPS. Plasma, DBS, or DPS that had a VL that was undetectable or lower than the LOD were classified as negative (i.e., <5,000 copies/ml or <1,000 copies/ml) for ARV TF.
All confidence intervals (CIs) are reported at the 95% confidence level.
Overall, the prevalences of ARV TF at a threshold of 5,000 copies/ml were 0.18 in plasma, 0.55 in DBS (P <0.001), and 0.19 in DPS (P = 0.38), while at the 1,000-copies/ml threshold, the prevalences of ARV TF were 0.23 in plasma, 0.80 in DBS (P <0.001), and 0.27 in DPS (P = 0.71) (Table 3).
TABLE 3.
Estimated prevalences of ARV TF in plasma, DBS, and DPS using the CAP/CTM
| Reason for VL testing and ARV TF diagnostic threshold used | Prevalence (95% CI) of TF with: |
P valuea |
|||
|---|---|---|---|---|---|
| Plasmab | DBSc | DPSd | Plasma-DBS | Plasma-DPS | |
| Overall | |||||
| 5,000 copies/ml | 0.18 (0.16–0.21) | 0.55 (0.51–0.58) | 0.19 (0.16–0.23) | <.001 | 0.38 |
| 1,000 copies/ml | 0.23 (0.21–0.26) | 0.80 (0.77–0.83) | 0.27 (0.23–0.31) | <.001 | 0.71 |
| Routine | |||||
| 5,000 copies/ml | 0.18 (0.15–0.22) | 0.51 (0.47–0.56) | 0.18 (0.14–0.22) | <.001 | 0.21 |
| 1,000 copies/ml | 0.22 (0.18–0.25) | 0.80 (0.76–0.82) | 0.25 (0.20–0.30) | <.001 | 0.28 |
| Targeted | |||||
| 5,000 copies/ml | 0.18 (0.14–0.22) | 0.58 (0.53–0.64) | 0.21 (0.16–0.26) | <.001 | 1 |
| 1,000 copies/ml | 0.25 (0.21–0.30) | 81.5 (0.77–0.86) | 0.30 (0.24–0.35) | <.001 | 1 |
Difference between prevalence estimates in plasma-DBS or plasma-DPS pairs tested for significance using McNemar's test for paired proportions.
n = 838.
n = 823.
n = 546.
DISCUSSION
Our study is the first to assess the programmatic utility of DBS-based screening for ARV TF with a commonly used VL assay according to the 2013 WHO ART guidelines. According to our results, inadequate measurement agreement and the low specificity and PPV of the CAP/CTM with DBS suggest that a large number of false-positive results for ARV TF would occur if DBS-based VL testing with the CAP/CTM were used. Since the main rationale for recommending routine VL monitoring in ART programs is to provide an early and accurate indication of ARV TF and the need to switch to second-line drugs, programmatic use of the CAP/CTM with DBS for ARV TF screening may lead to a substantial waste of resources and adverse clinical outcomes.
Using the 5,000-copies/ml diagnostic threshold recommended for DBS by the 2013 WHO guidelines, the CAP/CTM with DBS overestimated the prevalences of ARV TF by approximately 33% and 40% among patients receiving routine 6-month and targeted VL testing, respectively. In clinical practice, the 33% of patients who screened false positive for TF via routine monitoring would include additional unnecessary targeted VL testing, which would increase human resource demands and laboratory costs. Among the 40% of patients who screened false positive for TF via targeted DBS VL testing, two possible courses of action would likely occur. First, in countries with the available resources and infrastructure, HIV genotyping would be performed to confirm the diagnosed ARV TF. Second, in countries without access to genotyping, the current regimen would be unnecessarily switched to a second- or third-line regimen. The first scenario would increase laboratory testing costs, while the second scenario would lead to unnecessary regimen switching (to more expensive ARV drugs) and potentially adverse clinical outcomes.
The 2013 WHO guidelines stipulate that DBS-based VL testing use a higher threshold of 3,000 to 5,000 copies/ml for diagnosing ARV TF based on reports of reduced sensitivity of VL measurement in DBS at lower VL levels (10, 11). Our findings support recommendations to refrain from lowering the diagnostic threshold when DBS are used. However, according to our results, ARV TF screening in DBS using the CAP/CTM remains inadequate even at higher thresholds of 3,000 and 5,000 copies/ml.
Currently, there are eight conventional assays prequalified by the WHO for measuring VL in plasma, including versions of the Cobas (Roche), Versant (Siemens), NucliSENS (bioMérieux SA), and Abbott RealTime (Abbott) platforms and multiple automated or manual RNA extraction methods (19). The performance of each of these assays in measuring the VL in DBS and DPS has been evaluated in several studies, and the results have varied, especially for DBS. DBS-based VL testing has been associated with reduced sensitivity in several studies (12, 20, 21) but not in others (22, 23, 24, 25), with heterogeneity of results apparently relating to the RNA extraction methods and assays used. Marconi et al. found better agreement between DBS and plasma by using the Abbott mSample preparation system (m2000sp) (22), which is RNA specific, meaning that viral DNA is not coextracted and coamplified (as is done by the automated CAP/CTM extraction method that we used), which may explain the reduced difference between VL measurements in plasma and DBS using the Abbott platform.
Andreotti et al. found better agreement between DBS and plasma using the Cobas TaqMan analyzer when HIV RNA extraction was performed with the NucliSENS miniMAG system (bioMérieux, Marcy l'Etoile, France) instead of the High Pure system viral nucleic acid kit, which is the manual extraction method for the Roche Cobas TaqMan assay (23). Monleau et al. compared four manual RNA extraction methods from commercial VL assays and found that the RNA extraction method is an important factor in obtaining reliable RNA quantification and PCR amplification of HIV-1 on DPS/DBS, with some methods underestimating RNA recovery (20). In light of these conflicting results, the WHO recommendations for DBS-based VL monitoring should not treat different assay versions, types, and RNA extraction methods used with DBS as homogeneous. Explicit recommendations for VL testing with DBS for each of the prequalified VL assay versions and types should be included, or recommendations for VL testing with DBS should be further qualified until evidence for or against their use becomes conclusive.
The sensitivity, specificity, and predictive values of the CAP/CTM with DPS were all excellent, and the differences in ARV TF prevalence estimates between plasma and DPS were nonsignificant. Despite the excellent performance of DPS, their programmatic value for routine VL monitoring may be limited, at present, due to the complexity of specimen-processing requirements. However, recent advances in plasma separation technologies designed for point-of-care applications may be used for DPS-based VL monitoring. Liu et al. reported on a simple-to-use and low-cost membrane-based sedimentation-assisted plasma separator capable of separating a relatively large volume of plasma from undiluted whole blood within minutes (26). A novel microfluidic blood filtration element (BFE) that extracts plasma from whole blood in less than 10 min was demonstrated by Homsy et al. to be suitable for clinical application (27), and the use of red blood cell (RBC) agglutination for separating plasma from finger-prick volumes of whole blood directly in microfluid paper is also possible (28).
The low specificity and PPV of the CAP/CTM on DBS suggest that a large number of false-positive results for ARV TF may occur if this approach is used for routine VL monitoring. These results add to the published literature highlighting the suboptimal performance of DBS-based VL testing using the CAP/CTM. As such, recommendations for the use of DBS for routine VL monitoring may need to be further qualified. National ART programs currently considering how to adapt and implement the 2013 WHO guidelines should consider carefully the existing evidence and research gaps relating to use of DBS for routine VL monitoring.
ACKNOWLEDGMENTS
We acknowledge Roche Products (Pty) Ltd. (Diagnostics Division, South Africa) for donating the VL test kits and DBS/DPS testing supplies. Roche Products had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
This research was partially supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention and the Namibia Institute of Pathology (NIP).
We declare no competing interests.
Use of trade names is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services, the Public Health Service, or the Centers for Disease Control and Prevention. The findings and conclusions in this report are ours and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 20 August 2014
REFERENCES
- 1.UNAIDS. 2013. Global update on HIV treatment 2013: results, impact and opportunities. http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/20130630_treatment_report_summary_en.pdf.
- 2.UNAIDS. 2012. Treatment 2015. http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/JC2484_treatment-2015_en.pdf.
- 3.PEPFAR. 2012. PEPFAR blueprint for an AIDS-free generation. http://www.pepfar.gov/documents/organization/201386.pdf.
- 4.World Health Organization. 2013. The 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 5.World Health Organization. 2010. The 2010 revision of WHO antiretroviral therapy for HIV infection in adults and adolescents. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 6.Calmy A, Ford N, Hirschel B, Reynolds SJ, Lynen L, Goemaere E, de la Vega FG, Perrin L, Rodriguez W. 2007. HIV viral load monitoring in resource-limited regions: optional or necessary? Clin. Infect. Dis. 44:128–134. 10.1086/510073. [DOI] [PubMed] [Google Scholar]
- 7.Stevens WS, Marshall TM. 2010. Challenges in implementing HIV load testing in South Africa. J. Infect. Dis. 201:S78–S84. 10.1086/650383. [DOI] [PubMed] [Google Scholar]
- 8.Mei JV, Alexander JR, Adam BW, Hannon WH. 2001. Use of filter paper for the collection and analysis of human whole blood specimens. J. Nutr. 131:S1631–S1636. [DOI] [PubMed] [Google Scholar]
- 9.Brambilla D, Jennings C, Aldrovandi G, Bremer J, Comeau AM, Cassol SA, Dickover R, Jackson JB, Pitt J, Sullivan JL, Butcher A, Grosso L, Reichelderfer P, Fiscus SA. 2003. Multicenter evaluation of use of dried blood and plasma spot specimens in quantitative assays for human immunodeficiency virus RNA: measurement, precision, and RNA stability. J. Clin. Microbiol. 41:1888–1893. 10.1128/JCM.41.5.1888-1893.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johannessen A, Garrido C, Zahonero N, Sandvik L, Naman E, Kivuyo SL, Kasubi MJ, Gundersen SG, Bruun JN, de Mendoza C. 2009. Dried blood spots perform well in viral load monitoring of patients who receive antiretroviral treatment in rural Tanzania. Clin. Infect. Dis. 49:976–981. 10.1086/605502. [DOI] [PubMed] [Google Scholar]
- 11.Garrido C, Zahonero N, Corral A, Arredondo M, Soriano V, de Mendoza C. 2009. Correlation between human immunodeficiency virus type 1 (HIV-1) RNA measurements obtained with dried blood spots and those obtained with plasma by use of NucliSENS EasyQ HIV-1 and real time HIV load tests. J. Clin. Microbiol. 47:1031–1036. 10.1128/JCM.02099-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan H, Seth A, Rainen L, Fernandes H. 2010. Coamplification of HIV-21 proviral DNA and viral RNA in assays used for quantification of HIV-1 RNA. J. Clin. Microbiol. 48:2186–2190. 10.1128/JCM.02034-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marconi A, Balestrieri M, Comastri G, Pulvirenti FR, W. Gennari W, Tagliazucchi S, Pecorari M, Borghi V, Marri D, Zazzi M. 2009. Evaluation of the Abbott real-time HIV-1 quantitative assay with dried blood spot specimens. Clin. Microbiol. Infect. 15:93–97. 10.1111/j.1469-0691.2008.02116.x. [DOI] [PubMed] [Google Scholar]
- 14.Namibia Ministry of Health and Social Services. 2010. National guidelines for ART, 3rd ed. Namibia. [Google Scholar]
- 15.World Health Organization. 2012. WHO Prequalification of Diagnostics Programme, public report the COBAS Ampliprep/COBAS TaqMan (analyzer HIV-1 Test V. 2.0 (Roche)) for HIV-1. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 16.Schumacher W, Frick E, Kauselmann M, Maier-Hoyle V, van der Vliet R, Babiel R. 2007. Fully automated quantification of human immunodeficiency virus (HIV) type 1 RNA in human plasma by the COBAS Ampliprep/COBAS TaqMan system. J. Clin. Virol. 38:304–312. 10.1016/j.jcv.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Ouma KN, Basavaraju SV, Okonji JA, Williamson J, Thomas TK, Mills LA, Nkengasong JN, Zeh C. 2013. Evaluation of quantification of HIV-1 RNA viral load in plasma and dried blood spots by use of the semiautomated Cobas Amplicor assay and the fully automated Cobas Ampliprep/TaqMan assay, version 2.0, in Kisumu, Kenya. J. Clin. Microbiol. 51:1208–1218. 10.1128/JCM.03048-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310. [PubMed] [Google Scholar]
- 19.Perez-Gonzalez M. 2013. WHO prequalification of diagnostics update, AMDS Annual Stakeholders and Partners Meeting. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 20.Monleau M, Montavon C, Laurent C, Segondy M, Montes B, Delaporte E, Boillot F, Peeters M. 2009. Evaluation of different RNA extraction methods and storage conditions of dried plasma or blood spots for human immunodeficiency virus type 1 RNA quantification and PCR amplification for drug resistance testing. J. Clin. Microbiol. 47:1107–1118. 10.1128/JCM.02255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waters L, Kambugu A, Tibenderana H, Meya D, John L, Mandalia S, Nabankema M, Namugga I, Quinn TC, Gazzard B, Reynolds SJ, Nelson M. 2007. Evaluation of filter paper transfer of whole-blood and plasma samples for quantifying HIV RNA in subjects on antiretroviral therapy in Uganda. J. Acquir. Immune Defic. Syndr. 46:590–593. 10.1097/QAI.0b013e318159d7f4. [DOI] [PubMed] [Google Scholar]
- 22.Marconi A, Balestrieri M, Comastri G, Pulvirenti FR, W. Gennari W, Tagliazucchi S, Pecorari M, Borghi V, Marri D, Zazzi M. 2009. Evaluation of the Abbott real-time HIV-1 quantitative assay with dried blood spot specimens. Clin. Microbiol. Infect. 15:93–97. 10.1111/j.1469-0691.2008.02116.x. [DOI] [PubMed] [Google Scholar]
- 23.Andreotti M, Pirillo M, Guidotti G, Ceffa S, Paturzob G, Germano P, Luhangac R, Chimwaza D, Mancini MG, Marazzi MC, Vella S, Palombi L, Giuliano M. 2010. Correlation between HIV-1 viral load quantification in plasma, dried blood spots, and dried plasma spots using the Roche Cobas TaqMan assay. J. Clin. Virol. 47:4–7. 10.1016/j.jcv.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Johannessen A, Garrido C, Zahonero N, Sandvik L, Naman E, Kivuyo SL, Kasubi MJ, Gundersen SG, Bruun JN, de Mendoza C. 2009. Dried blood spots perform well in viral load monitoring of patients who receive antiretroviral treatment in rural Tanzania, abstr V-136 16th Conference on Retroviruses and Opportunistic Infections. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz N, Pérez M, Díaz H, Izquierdo M, Blanco M, Machado L, Silva E. 2012. Validation of Cobas Ampliprep/Cobas TaqMan HIV-1 test on dried blood spots. J. Int. AIDS Soc. 15:18192. [Google Scholar]
- 26.Liu C, Mauk M, Gross R, Bushman FD, Edelstein PH, Collman RG, Bau HH. 2013. Membrane-based, sedimentation-assisted plasma separator for point-of-care applications. Anal. Chem. 85:10463–10470. 10.1021/ac402459h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Homsy A, van der Wal PD, Doll W, Schaller R, Korsatko S, Ratzer M, Ellmerer M, Pieber TR, Nicol A, de Rooij NF. 2012. Development and validation of a low cost blood filtration element separating plasma from undiluted whole blood. Biomicrofluidics. 6:12804–128049. 10.1063/1.3672188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang X, Forouzan O, Brown TP, Shevkoplyas SS. 2012. Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices. Lab Chip 12:274–280. 10.1039/c1lc20803a. [DOI] [PubMed] [Google Scholar]