Abstract
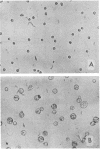
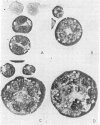
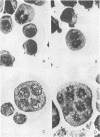
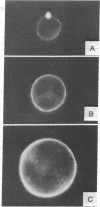
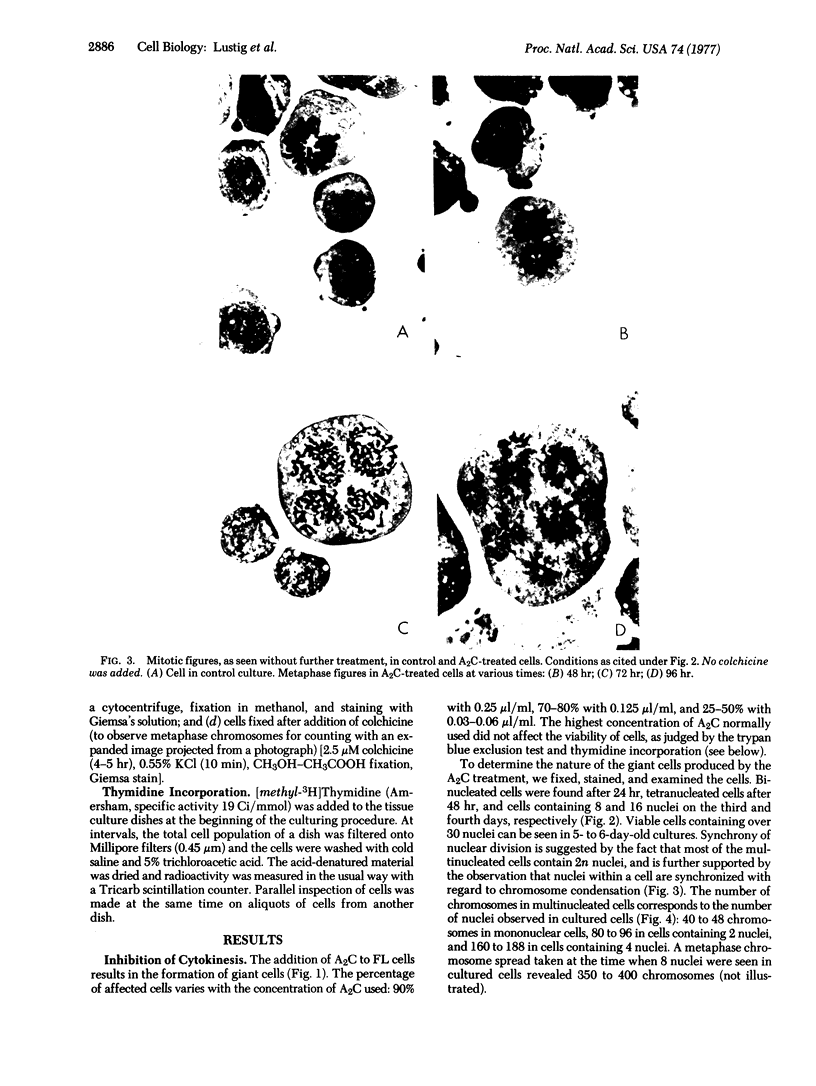
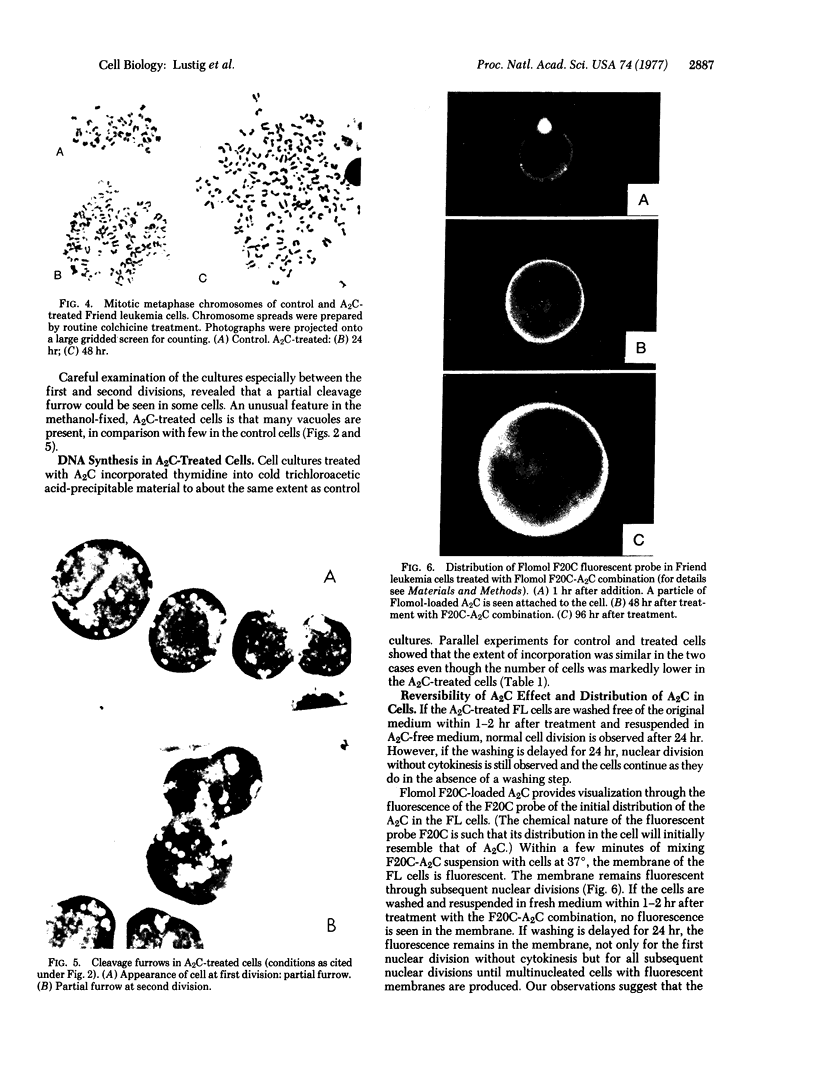
Treatment of a line of Friend leukemia cells with a dispersion of the membrane mobility agent, A2C, yields cells that undergo successive nuclear divisions without cytokinesis, resulting eventually in cells with as many as 30 nuclei. Neither the DNA replication rate of the cells nor the generation time is different after treatment; in addition, the multiple nuclei divide synchronously, and the chromosome number corresponds to the number of nuclei in the cell. Inhibition of cytokinesis is not observed if the cells are washed with reagent-free medium within 1 hr of treatment, but is observed if washing is delayed for 24 hr. Membrane mobility agent loaded with the fluorescent probe, Flomol F20C, leads to fluorescent membrane; fluorescence disappears from the membrane after a change of medium within 1 hr, but not after a change of medium within 24 hr. Some stages in the overall development resemble those seen for cytochalasin B inhibition of cytokinesis, although the mechanisms may well be different for the inhibition promoted by membrane mobility agent. The inhibition of cytokinesis by A2C provides a potentially interesting means of studying cytokinesis and the regulation of differentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beams H. W., Kessel R. G. Cytokinesis: a comparative study of cytoplasmic division in animal cells. Am Sci. 1976 May-Jun;64(3):279–290. [PubMed] [Google Scholar]
- Carter S. B. Effects of cytochalasins on mammalian cells. Nature. 1967 Jan 21;213(5073):261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- Davis F. M., Adelberg E. A. Use of somatic cell hybrids for analysis of the differentiated state. Bacteriol Rev. 1973 Jun;37(2):197–214. doi: 10.1128/br.37.2.197-214.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier R. E., Pardee A. B. Cell cycle studies of mononucleate and cytochalasin-B--nduced binucleate fibroblasts. Proc Natl Acad Sci U S A. 1975 Mar;72(3):869–873. doi: 10.1073/pnas.72.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. R. Analysis of erythropoeisis at the molecular level. Nature. 1976 Jul 29;262(5567):353–356. doi: 10.1038/262353a0. [DOI] [PubMed] [Google Scholar]
- Hatzfeld J., Buttin G. Temperature-sensitive cell cycle mutants: a chinese hamster cell line with a reversible block in cytokinesis. Cell. 1975 Jun;5(2):123–129. doi: 10.1016/0092-8674(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Hirano A., Kurimura T. Virally transformed cells and cytochalasin B. I. The effect of cytochalasin B on cytokinesis, karyokinesis and DNA synthesis in cells. Exp Cell Res. 1974 Nov;89(1):111–120. doi: 10.1016/0014-4827(74)90193-1. [DOI] [PubMed] [Google Scholar]
- Hoehn H., Sprague C. A., Martin G. M. Effects of cytochalasin B on cultivated human diploid fibroblasts and its use for the isolation of tetraploid clones. Exp Cell Res. 1973 Jan;76(1):170–174. doi: 10.1016/0014-4827(73)90432-1. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Rao P. N. Nucleo-cytoplasmic interactions in the acheivement of nuclear synchrony in DNA synthesis and mitosis in multinucleate cells. Biol Rev Camb Philos Soc. 1971 Feb;46(1):97–155. doi: 10.1111/j.1469-185x.1971.tb01180.x. [DOI] [PubMed] [Google Scholar]
- Kosower E. M., Kosower N. S., Faltin Z., Diver A., Saltoun G., Frensdorff A. Membrane mobility agents. A new class of biologically active molecules. Biochim Biophys Acta. 1974 Sep 6;363(2):261–266. doi: 10.1016/0005-2736(74)90065-0. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Kosower E. M., Wegman P. Membrane mobility agents. II. Active promoters of cell fusion. Biochim Biophys Acta. 1975 Sep 2;401(3):530–534. doi: 10.1016/0005-2736(75)90250-3. [DOI] [PubMed] [Google Scholar]
- Krishan A., Ray-Chaudhuri R. Asynchrony of nuclear development in cytochalasin-induced multinucleate cells. J Cell Biol. 1969 Dec;43(3):618–621. doi: 10.1083/jcb.43.3.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Lustig S., Pluznik D. H., Kosower N. S., Kosower E. M. Membrane mobility agent alters the consequences of lectin-cell interaction in a malignant cell membrane. Biochim Biophys Acta. 1975 Sep 2;401(3):458–467. doi: 10.1016/0005-2736(75)90243-6. [DOI] [PubMed] [Google Scholar]
- Preisler H. D., Lyman G. Differentiation of erythroleukemia cells in vitro: properties of chemical inducers. Cell Differ. 1975 Jun;4(3):179–185. doi: 10.1016/0045-6039(75)90039-1. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Cytokinesis in animal cells. Int Rev Cytol. 1971;31:169–213. doi: 10.1016/s0074-7696(08)60059-5. [DOI] [PubMed] [Google Scholar]
- Reuben R. C., Wife R. L., Breslow R., Rifkind R. A., Marks P. A. A new group of potent inducers of differentiation in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):862–866. doi: 10.1073/pnas.73.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. J., Wigglesworth N. M. Cell line which is temperature-sensitive for cytokinesis. J Cell Physiol. 1972 Oct;80(2):253–259. doi: 10.1002/jcp.1040800212. [DOI] [PubMed] [Google Scholar]