Abstract
Stem cell-derived cardiomyocytes (CMs) are often electrophysiologically immature and heterogeneous, which represents a major barrier to their in vitro and in vivo application. Therefore, the purpose of this study was to examine whether Neuregulin-1β (NRG-1β) treatment could enhance in vitro generation of mature “working-type” CMs from induced pluripotent stem (iPS) cells and assess the regenerative effects of these CMs on cardiac tissue after acute myocardial infarction (AMI). With that purpose, adult mouse fibroblast-derived iPS from α-MHC-GFP mice were derived and differentiated into CMs through NRG-1β and/or dimethyl sulfoxide (DMSO) treatment. Cardiac specification and maturation of the iPS was analyzed by gene expression array, quantitative real-time polymerase chain reaction, immunofluorescence, electron microscopy, and patch-clamp techniques. In vivo, the iPS-derived CMs or culture medium control were injected into the peri-infarct region of hearts after coronary artery ligation, and functional and histology changes were assessed from 1 to 8 weeks post-transplantation. On differentiation, the iPS displayed early and robust in vitro cardiogenesis, expressing cardiac-specific genes and proteins. More importantly, electrophysiological studies demonstrated that a more mature ventricular-like cardiac phenotype was achieved when cells were treated with NRG-1β and DMSO compared with DMSO alone. Furthermore, in vivo studies demonstrated that iPS-derived CMs were able to engraft and electromechanically couple to heart tissue, ultimately preserving cardiac function and inducing adequate heart tissue remodeling. In conclusion, we have demonstrated that combined treatment with NRG-1β and DMSO leads to efficient differentiation of iPS into ventricular-like cardiac cells with a higher degree of maturation, which are capable of preserving cardiac function and tissue viability when transplanted into a mouse model of AMI.
Introduction
Although numerous studies have demonstrated the benefit of stem cell therapy in cardiovascular disease, positive outcomes have primarily been mediated by protective rather than regenerative effects (reviewed in [1]). Thus, identifying a reliable source of cardiovascular progenitors, which are capable of repopulating injured myocardium, represents a major goal in regenerative medicine. In this regard, the lack of stem cell populations [2,3] with true cardiac differentiation potential has remained a barrier to effective cardiac therapy. So far, the main sources employed for generating cardiomyocytes (CMs) have been adult cardiac progenitors and embryonic stem cells (ESCs) [4,5]. However, there are technical limitations associated with effectively identifying and isolating heart progenitors that are capable of robust in vivo cardiac differentiation, and emerging immunogenic, tumorigenic, and ethical concerns have restricted the clinical use of ESCs.
Induced pluripotent stem (iPS) cells, which are generated by reprogramming somatic cells with a set of overexpressed ESC-related transcription factors, were recently discovered as a potentially valuable source of stem cells for cardiac regeneration [6]. Notably, the use of iPS cells can circumvent certain ESC-associated limitations, such as immunogenicity. Nevertheless, complications that are inherent to pluripotency, such as tumorigenic potential, still represent a major hurdle in the clinical application of iPS cells; however, these issues could be overcome through proper in vitro differentiation and selection protocols (reviewed in [7]). Although iPS cell-derived CMs (iPS-CMs) have been generated both in vitro [8,9] and in vivo, using murine and swine models of myocardial infarction [10,11], the resulting CMs have displayed an immature fetal-like phenotype that is similar to ESC-derived CMs [12]. Therefore, in order to achieve successful cardiac tissue regeneration, differentiation protocols will need to be optimized to produce homogenous cultures of mature cardiac cells, preferably exhibiting a “working” phenotype.
Neuregulin-1 (NRG-1) is a protein that belongs to the epidermal growth factor family and functions as a key regulator of both cardiac development and postnatal function (reviewed in [13]). In response to stress, microvascular endothelial cells in the myocardium produce NRG-1 [14], which binds to ErbB-4 receptor on CMs. This results in dimerization of ErbB-4 with ErbB-2, and subsequent signaling to promote key cellular responses, such as survival and proliferation, in neonatal [15] and adult CMs (ACMs) [15,16]. In vivo, NRG-1 was found to be involved in structural preservation of the myocardium [14,17] as well as in angiogenic [18] and anti-atherosclerotic effects [19]. Moreover, under pathological conditions, NRG-1 mediated positive effects on heart function and survival [20,21]. In addition, NRG-1 has been used to stimulate stem cell differentiation into cardiac cells. This is in line with the fact that NRG-1 induces differentiation of cardiac precursors into cells of the cardiac conduction system during embryonic development [22]. In this regard, NRG-1 was also found to modulate the ratio of nodal to “working-type” cells on ESC differentiation in vitro [23].
In view of the cardiomyogenic role of NRG-1, we hypothesized that NRG-1β might drive differentiation of iPS cells into more mature and functional CMs, which could be useful for regenerative therapies. In this study, we have demonstrated that in vitro NRG-1β treatment effectively induced differentiation of adult mouse fibroblast-derived iPS cells into CMs with a ventricular phenotype with more mature electrophysiological properties. These NRG-1β-generated CMs could be exploited for in vivo transplantation, contributing to regeneration of injured cardiac tissue after acute myocardial infarction (AMI).
Materials and Methods
Generation and characterization of murine iPS cells
Murine iPS cells were generated from adult fibroblasts, which were derived from female αMHC-GFP transgenic mice, in which green fluorescent protein is driven by the myosin heavy chain promoter (donated by Dr. L.J. Field, Indianapolis University). Fibroblasts were reprogrammed through retroviral transduction of pluripotent factors (Oct3/4, Sox2, and Klf4) as described [6]. Stem cell clones were maintained in standard iPS media, which consisted of Dulbecco's modified Eagle medium with high glucose (DMEM HG; Gibco), 15% knock-out serum replacement (Gibco), 1% non-essential amino acids (BioWhittaker), 1% penicillin–streptomycin (BioWhittaker), 1% l-glutamine (Gibco), 0.1 mM β-mercaptoethanol (Gibco), and 103 U/mL leukemia inhibitory factor (Chemicon).
Quantitative real-time polymerase chain reaction
Total RNA was isolated from undifferentiated and differentiated cells using the Ultraspec Total RNA Isolation Kit (Biotecx Laboratories), and 1 μg was used to synthesize cDNA using the Superscript II Reverse Transcription Kit (Invitrogen) according to the manufacturer's protocol. The quantitative real-time polymerase chain reaction (qRT-PCR) reactions were carried out using the 7300 Real-Time PCR System (Applied Biosystems).
Alkaline phosphatase staining and immunofluorescence
Alkaline phosphatase staining was performed using the Leukocyte Alkaline Phosphatase Kit (Sigma) according to the manufacturer's protocol. For immunofluorescence detection of pluripotency markers, iPS cells were fixed with Zn-Formalin (Thermo Scientific) and permeabilized with 0.1% Triton X-100 for 30 min at room temperature (R/T). Rabbit antibodies against Oct3/4 (Santa Cruz Biotechnology) and Nanog (Abcam) were diluted (1:50 and 1:100, respectively) in phosphate-buffered saline (PBS) with 3% bovine serum albumin (BSA; Nacalai Tesque) and incubated for 30 min at 37°C. Cy3-conjugated secondary antibodies (Sigma) were diluted (anti-rabbit IgG Cy3-conjugated; 1:1,500 and anti-mouse IgG Cy3-conjugated; 1:1,000) in 3% BSA/PBS and incubated for 30 min at 37°C in the dark. TOPRO-3 (Molecular Probes) was added for nuclear staining. For immunofluorescence detection of cardiac markers, differentiated cells were fixed with Zn-Formalin, permeabilized with 0.1% Tween (Sigma), and blocked with 0.4% Fish Gelatin (Sigma) for 1 h, at R/T. Next, the cells were incubated for 2 h, at R/T with the following primary antibodies: mouse monoclonal anti-Actinin (1:100; Sigma-Aldrich), rabbit anti-Connexin 43 (Cx43; 1:500; Sigma-Aldrich), goat anti-GATA4 (1:50; Santa Cruz Biotechnology), mouse monoclonal troponin (1:300; Abcam), and mouse monoclonal anti-Titin (1:300; Developmental Studies Hybridoma Bank). Secondary antibodies (1:500) were incubated at R/T for 1 h in the dark and included: Alexa Fluor-594 goat anti-mouse (Invitrogen), Alexa Fluor-594 donkey anti-rabbit (Invitrogen), and Cy3 donkey anti-goat (Jackson ImmunoResearch). TOPRO-3 was used for nuclear staining. All images were obtained with a Zeiss LSM 510 META laser confocal microscope (Carl Zeiss) and were analyzed using AIM 4.2 (Carl Zeiss).
Teratoma formation
To test the pluripotency of iPS cells in vivo, 1×106 cells were subcutaneously transplanted into the dorsal flanks of 6–8-week-old Rag2−/−γc−/− male mice (generously donated by Dr. Spits, Academic Medical Center of Amsterdam) (two animals/iPS clone). Adult fibroblasts from αMHC-GFP transgenic mice (used to generate the iPS cells) and non-irradiated mouse embryonic fibroblasts (MEFs) were injected as negative controls, while the D3 mouse ESC line was injected as a positive control. Six to eight weeks later, developed tumors were dissected, fixed in 10% formalin (Thermo Scientific) for 24 h, and embedded in paraffin. Samples were then sectioned and stained with hematoxylin-eosin.
In vitro cardiac differentiation of iPS cells
For cardiac differentiation, iPS cells were plated onto 0.1% gelatin-coated dishes to eliminate any contaminating MEFs. Two days later, iPS colonies were flushed and cultured on Ultra Low Attachment Culture Dishes (Corning) to allow embryoid body (EB) formation (day 5). EBs were maintained in suspension for 5 days in differentiation media, which consisted of DMEM HG (Gibco) supplemented with 10% fetal bovine serum (FBS; Biochrom), 1% l-glutamine (Gibco), and 1% penicillin–streptomycin (BioWhittaker). EBs were then plated on gelatin-coated dishes with differentiation media (considered as day 0) and treated with 1% dimethyl sulfoxide (DMSO; Sigma-Aldrich) and/or 100 ng/mL of NRG-1β (ImmunoTools) on days 1, 3, and 5. One week later, the media was replaced with cardiac cell-specific media (Claycomb media; Sigma-Aldrich) supplemented with 10% FBS (Biochrom), 1% l-glutamine (Gibco), and 1% penicillin–streptomycin (BioWhittaker). To determine cardiac differentiation, GFP+ areas (indicative of cardiac specific αMHC expression) were quantified. Twenty random pictures were taken with a 2.5× objective (at day 7 and 14) and quantified using the Analysis FIVE software (Olympus Biosystems GmbH). Data were expressed as the percentage of GFP+ areas.
Transmission electron microscopy
Isolated iPS cell-derived CMs were fixed in 3.5% glutaraldehyde for 1 h at 37°C, post-fixed in 2% OsO4, and stained in 2% uranyl acetate for 2 h at 4°C (dark). Serial semi-thin (1.5 μm) sections were cut using an Ultracut UC-6 (Leica), mounted onto slides, and stained with 1% toluidine blue. In addition, ultrathin sections (0.06–0.09 μm) were prepared with the Ultracut and stained with lead citrate. Mouse hearts were cut into slices (1.0 mm) and fixed with Zn-Formalin/0.5% glutaraldhehyde at 4°C overnight (o/n). For immunogold staining, samples were incubated with chicken anti-GFP antibody [diluted 1:200 in Blocking Solution I (see Supplementary Materials and Methods; Supplementary Data are available online at www.liebertpub.com/scd); Aves Labs] for 3 days at 4°C with mild agitation, followed by a 24-h incubation (R/T under mild agitation) with colloidal gold-conjugated goat anti-chicken [diluted 1:50 in Blocking Solution II (see Supplementary Materials and Methods); UltraSmall; Aurion]. To stabilize the silver particles, samples were immersed in 0.05% gold chloride (10 min at 4°C) and post-fixed in 2% glutaraldehyde (30 min). Finally, semi-thin sections were prepared (1.5 μm), selected under a light microscope, and re-embedded for ultra-thin sections at 70 nm. All photomicrographs were obtained using an FEI Tecnai G2 Spirit transmission electron microscope (FEI Europe) with a Morada digital camera (Olympus Soft Image Solutions GmbH).
Action potential recordings
At day 7 and 14 of differentiation, beating CMs were examined on an inverted fluorescence microscope (Axiovert 200; Zeiss), which enabled the identification of GFP+ cells. To further evaluate the CMs, glass microelectrodes filled with 3 mol/L KCl (resistance: 20–30 MΩ) were used to record intracellular action potentials (APs). All recordings were performed at 37°C. Neonatal CMs (NCMs) were included as control group. Signals were amplified by an SEC-10LX single-electrode clamp amplifier (NPI Electronic; http://npielectronic.com/) and acquired with PULSE software (HEKA, htpp://heka.com/). AP parameters were analyzed off-line using Mini Analysis software (Synaptosoft, http://synaptosoft.com/).
Array data analysis
Total RNA was extracted from cells using the Ultraspec Total RNA Isolation Kit and was hybridized to a GeneChip® Human Gene 2.0 ST Array. Samples were normalized with RMA [24], and probesets with low expression were filtered with R/Bioconductor [25]. In the filtering process, probesets with an expression value lower than 5 in all the analyzed samples were discarded. Fold changes (FC) for the remaining 27,028 probesets were calculated, and a logFC threshold of 1 was established to select differentially expressed genes.
The functional enrichment analysis of Gene Ontology categories [26] was performed with the selected genes using the hypergeometric distribution in R [25]. The biological knowledge extraction was complemented through the use of Ingenuity Pathway Analysis (Ingenuity Systems, www.ingenuity.com), the database that includes manually curated and fully traceable data derived from literature sources. The unsupervised hierarchical correlation clustering analysis was performed in R [25] using all probes included on the array.
Different groups of samples were compared in order to evaluate induction of cardiac differentiation, cardiac maturation, as well as differences among differentiation treatments. The data sets are available in the Gene Expression Omnibus database (http://ncbi.nlm.nih.gov/gds) under the accession number (GSE56511).
Surgical animal procedure and study design
Eight-week-old female DBA/2J mice (n=28) were obtained from Charles River Laboratories and underwent permanent coronary artery ligation as previously described [27]. Ten to fifteen minutes after artery ligation, iPS-CMs (day 7 of differentiation, 2×105 cells in 10 μL of DMEM) or control medium (10 μL) were injected into four points at the peri-infarct region using a Hamilton syringe (Hamilton, 701N; 10 μL). The survival rate of the course of the experiment was more than 90%. Only those animals that survived (n=26) and with an ejection fraction below 40% (as determined by echocardiography) at day 2 post-transplantation were included in the functional study (n=16; eight/group). For histological assessment at short term, two animals/group were included in the study. All experiments were performed in accordance with the principles of laboratory animal care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals from the Institute of Laboratory Animal Resources (Commission on Life Science, National Research Council). The University of Navarra Institutional Committee on Care and Use of Laboratory Animals also approved all animal procedures.
Echocardiography
Echocardiography was performed using a Vevo 770 ultrasound system (Visualsonics), and measurements were optimized for small animals and performed as previously described [28]. Echocardiography was performed at 2, 30, and 60 days after ligation of the left anterior descending artery. Left ventricular (LV) remodeling was quantified according to the guidelines and standards set by the American Society of Echocardiology, the Guide to Micro-Echocardiography Study using the Vevo770, and the Vevo 770® Protocol-Based Measurements and Calculations guide.
Tissue processing and staining
Animals were sacrificed for histological analysis at 1, 2, and 4 weeks (two mice/group) as well as at 2 months (eight animals/group). Mice were anesthetized, injected with 100 μL of 0.1 mM cadmium chloride (Sigma) for diastole cardiac arrest, and perfusion fixed for 15 min with Zn-Formalin (Thermo Scientific) under physiological pressure. The hearts were excised, fixed o/n in Zn-Formalin at 4°C, cut into three equally sized blocks (apical, mid-ventricular, and basal), dehydrated in 70% ethanol (4°C, o/n), and embedded in paraffin. For histological analysis, 5 μm serial sections were prepared. The iPS-CMs were identified through immunohistochemistry using either rabbit anti-GFP (Invitrogen) or chicken anti-GFP (Abcam) diluted 1:500 in Tris-buffered saline (TBS: Tris 50 mM, NaCl 0.9%, pH 7.36). Immunofluorescence was performed using antibodies against caveolin-1 (1:125; Cell Signaling), alpha-smooth muscle actin (α-SMA; Cy3-conjugated, 1:500; Sigma), Cx43 (1:500; Sigma), and troponin (1:100; Abcam). In addition, the following secondary reagents were utilized: fluorescein isothiocyanate-conjugated donkey anti-rabbit (1:200; Jackson Immunoresearch), Alexa Fluor-633 rabbit anti-mouse IgG (1:400; Invitrogen), Alexa Fluor-594 goat anti-rabbit IgG (1:500; Invitrogen), and Alexa Fluor-488 goat anti-chicken (1:500; Invitrogen). For immunofluorescence, all primary and secondary antibodies were diluted in TBS. The EnVision+System™ (horseradish peroxidase-conjugated; Dako) was used for detection in immunohistochemistry. For confocal microscopy, an LSM 510 META microscope (Carl Zeiss) was used and images were analyzed with AIM 4.2 (Carl Zeiss). For Sirius Red staining, sections were deparaffinized and immersed for 90 min in 0.1% Fast Red (Sigma), which was diluted in a saturated solution of picric acid. They were then differentiated for 2 min in 0.01 N HCl (Sigma), dehydrated, and mounted in DPX.
Morphometric analysis
Infarct size and degree of tissue fibrosis were determined using the Sirius Red stained sections. Infarct size was assessed by quantifying images from 12 serial heart sections, 50 μm apart. Images were analyzed with AnalySIS software, and data were expressed as a percentage of the ischemic area versus the total LV area. For quantifying fibrosis, 24 images from serial heart sections of the peri-infarct zone were analyzed using AnalySIS software. Data were expressed as a percentage of the fibrotic area (red) within the peri-infarct zone versus the total tissue area.
Vascular density was quantified in animals that were sacrificed 2 months post-implantation. For capillary density (capillaries/mm2), 12 serial sections (50 μm apart) were stained with anti-caveolin-1, and vessels with diameters between 5 and 15 μm were quantified in the peri-infarct border. The arteriolar/arteries area (μm2/mm2) was quantified in a similar manner after staining with anti-α-SMA. Images were analyzed using software, which was developed with the Matlab platform.
Statistical analysis
Normal distribution was assessed using the Shapiro–Wilk test. All data were expressed as mean±standard deviation. In the case of normal distributions, comparisons between groups were performed using student's t-test or analysis of variance, followed by Bonferroni's and Tukey's HSD post-hoc tests. Non-parametric analyses were performed using Kruskal–Wallis and Mann–Whitney U test. Statistical analyses were performed using either Prism GraphPad 4.0 or SPSS 11.0 software. Differences were considered statistically significant when P<0.05.
Results
Generation and characterization of iPS cells
iPS cells were derived based on the protocol by Dr. Yamanaka. Tail fibroblasts were isolated from adult αMHC-GFP transgenic mice and retrovirally transduced with pluripotent genes (Sox2, Oct3/4, and Klf4). Approximately 3 weeks after infection, several clones began forming compact clusters with an ESC-like morphology. One week later, these clones were collected and expanded over a feeder layer of MEFs. Six independent, stable clones were isolated for both in vitro and in vivo characterization (Supplementary Figs. S1 and S2). These iPS cell clones consistently expressed several pluripotent genes (ie, Oct3/4, Klf4, Sox2, Nanog, c-Myc, Ecat1, Eras, Fbx15, and Rex1) as detected by qRT-PCR. Moreover, Oct3/4 and Nanog protein expression was confirmed by immunofluorescence in all six clones, which were also positive for alkaline phosphatase activity. Furthermore, the iPS cell clones efficiently generated teratomas when transplanted into immunosuppressed mice (Rag2−/−γc−/−), demonstrating their pluripotency (Supplementary Fig. S3).
iPS cells show robust cardiac differentiation potential
To examine cardiac differentiation potential, EBs were generated from the six iPS cell clones and differentiated during 14 days by treatment with 1% DMSO (Supplementary Fig. S4), which we have previously found to potentiate the cardiac differentiation of the mouse ESC cell line R1 (data not shown) and the mouse embryonal carcinoma cell line P19. We observed that iPS clone 1 and 2 (iPS-1 and iPS-2) displayed the most robust cardiac differentiation potential, producing larger GFP+ beating areas than the other clones (Supplementary Fig. S5A). Therefore, these two clones were selected for further studies. After 14 days, we identified upregulated expression of several cardiac-related genes, including early (Gata4 and Mef2c) and late (βMhc, αMhc, Mlc2v, and cardiac Actinin) markers (Supplementary Fig. S5B). We also detected the expression of various genes for cardiac-specific channels, such as L-type calcium channel (Cacna1c), ryanodine receptor (Ryr2), potassium/sodium channel (Hcn1), and phospholamban (Pln) (Supplementary Fig. S5B). As expected, decreased expression of pluripotency associated factors was observed after iPS cell differentiation (Supplementary Fig. S6). In addition, expression of cardiac-specific proteins (GATA4, Titin, Actinin, and Cx43) was observed in GFP+ cells through immunofluorescence at day 14 of differentiation (Supplementary Fig. S5C). Finally, generation of mature CMs was confirmed at the ultrastructural level by transmission electronic microscopy, which revealed typical sarcomeric organization with aligned and occasionally ramified myofibrils throughout the cytoplasm. In addition, abundant large mitochondria were found (Supplementary Fig. S5D).
NRG-1β potentiates cardiac differentiation in iPS cells
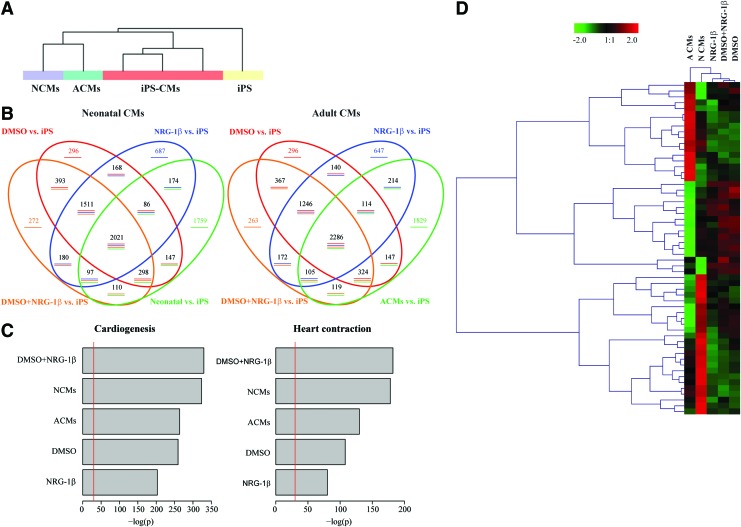
NRG-1β is known to be involved in numerous cardiac specific processes, including differentiation of ESCs into CMs. Therefore, the effect of NRG-1β during cardiac specification was examined using the iPS-1 clone. Thus, iPS cells were differentiated during 14 days with DMSO in the presence or the absence of NRG-1β. First, in an initial screening, the gene expression profiles of differentiated iPS-CMs with the different treatments were compared with their parental iPS cells and with ACMs and NCMs in order to determine whether the NRG-1β and/or DMSO treatment was related to cardiac differentiation. An unsupervised normalized clustering analysis classified the samples into three main groups: parental undifferentiated iPS cells, CMs (ACM and NCM), and iPS-CMs differentiated by the three treatments (NRG-1β, NRG-1β plus DMSO, or DMSO alone) (Fig. 1A). We observed 8,970 probes differentially expressed (logFC >1) between iPS-CMs and CMs (both ACMs and NCMs) in comparison with the undifferentiated iPS cells, with a similar distribution pattern when compared individually (Fig. 1B). In particular, a total of 1,974 probes (22%) were shared within all the groups, with almost half of the differentially expressed genes (3,532 probes) commonly expressed within the iPS-CMs independently of the treatment, indicating a robust cell differentiation. However, some differences at the gene expression level were observed between the iPS-CMs and CMs (Fig. 1B). Interestingly, the analysis of genes differentially expressed showed an enrichment in cardiogenesis and heart contraction categories in all iPS-CMs groups (Fig. 1C). One third of those genes were commonly shared between CMs and iPS-CMs in both categories (Supplementary Fig. S7) and more interestingly, CM differentiation with the combined treatment with NRG-1β plus DMSO enhanced the enrichment in cardiac categories, indicating proper cardiac differentiation (Fig. 1C). As expected, some differences between treatments and ACMs and/or NCMs were also observed. On the other hand, the expression of the pluripotency associated factors showed the opposite pattern, with most of the genes downregulated after differentiation (Supplementary Figs. S6 and S8). A more detailed analysis of cardiac-related categories between iPS-CMs, ACMs, and NCMs showed that DMSO and/or NRG-1β treatment induced a gene expression profile closer to NCMs (Fig. 1D) and significantly different from the parental iPS cells (Supplementary Figs. S6 and S8). Furthermore, the expression of those cardiac-specific genes differentially expressed in the iPS-CMs was validated by qRT-PCR and compared with NCMs. NRG-1β treatment induced a significantly higher expression of cardiac-specific genes, including Gata4, Gata6, αMhc, Myhl7, Myl3, cTnnc1, Ryr2, and Serca2a (Supplementary Fig. S9) and, importantly, induced a significantly higher expression of genes that are specific for fatty acids cycle such as Pdk4 and CD36 but significantly lower expression of glycolysis-related genes such as Slc2a1 and Slc2a4, indicating the formation of more mature cardiac cells (Supplementary Fig. S9).
FIG. 1.
Gene expression analysis of induced pluripotent stem-cardiomyocytes (iPS-CMs). (A) Unsupervised clustering of the samples: iPS-derived CMs (iPS-CMs) differentiated after treatment with dimethyl sulfoxide (DMSO) and/or neuregulin-1β (NRG-1β), neonatal CMs (NCMs), adult CMs (ACMs), and undifferentiated iPS cells (iPS). (B) Venn's diagram representing the differentially expressed genes (logFC >1) over parental iPS cells, in iPS-CMs and NCMs (left) and iPS-CMs and ACMs (right). (C) Biological function enrichment of the differentially expressed genes in cardiac categories (cardiogenesis and heart contraction) in iPS-CMs and CMs in comparison with parental iPS cells. (D) Heatmap and clustering of the differential gene expression observed between ACMs and NCMs in cardiac categories along with the iPS-CMs. Color images available online at www.liebertpub.com/scd
Furthermore, at the protein level, at day 7 of differentiation, the percentage of GFP+ area observed after NRG-1β and DMSO co-treatment was significantly greater than seen with either of the treatments alone [%GFP+ areas: DMSO: 17.7±3.0% (P<0.001 vs. NRG-1β) NRG-1β: 10.3±3.1%; DMSO+NRG-1β: 25.0±3.9% (P<0.01 vs. DMSO and P<0.001 vs. NRG-1β)] (Fig. 2). A similar effect was observed at day 14, with a significant increase in the number of GFP+ areas observed in the group treated with both DMSO and NRG-1β [%GFP+ areas: DMSO: 37.0±4.3% (P<0.001 vs. NRG-1β), NRG-1β: 19.8±4.4%; DMSO+NRG-1β: 44.7±5.7% (P<0.05 vs. DMSO and P<0.001 vs. NRG-1β)] (Fig. 2). Confirming these results, the combined treatment also significantly increased the expression of troponin-positive cells in comparison with the treatments alone, demonstrating the synergic effect of the NRG-1β and DMSO in cardiac differentiation [%cTNT+ areas: DMSO: 34.7±8.0% (P<0.01 vs. NRG-1β) NRG-1β: 20.5±11.1%; DMSO+NRG-1β: 39.8±10.5% (P<0.05 vs. DMSO and P<0.001 vs. NRG-1β)] (Supplementary Fig. S10).
FIG. 2.
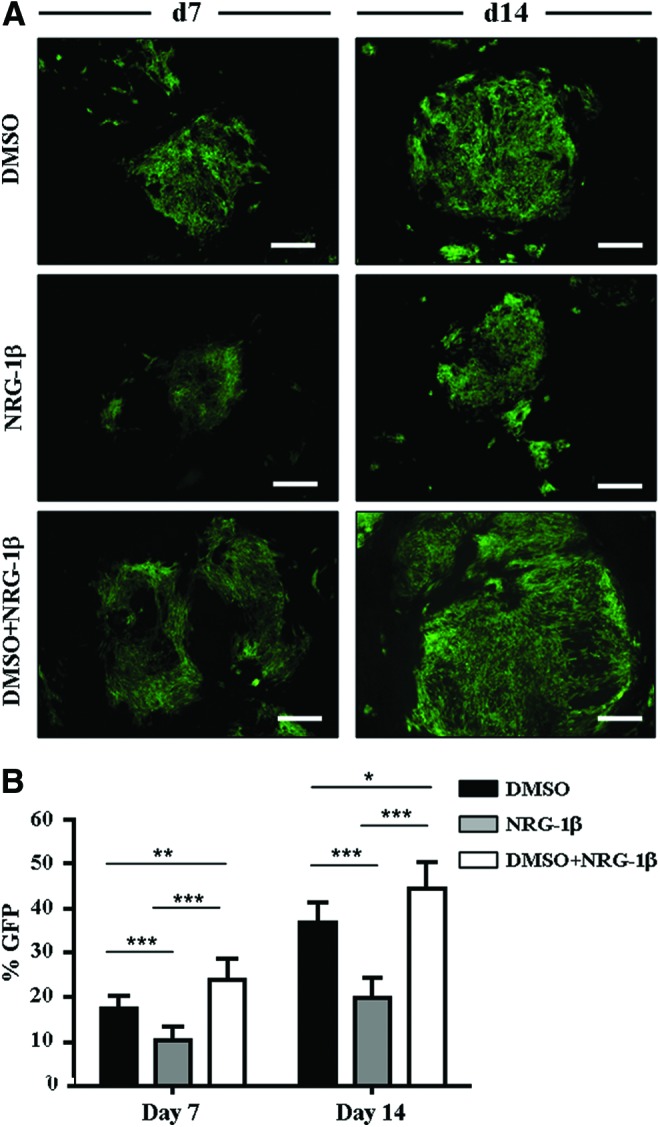
NRG-1β potentiates cardiac differentiation in iPS cells. (A) Differentiation of iPS-1 was induced with DMSO and/or NRG-1β. GFP+ clusters were detected at day 7 and 14 of differentiation, with larger GFP areas observed with combined treatment. Scale bars: 375 μm. (B) GFP+ areas were quantified at day 7 and 14 of differentiation. The percentage of GFP+ clusters was significantly higher when iPS cells were co-treated with DMSO and NRG-1β compared with either treatment alone (*P<0.05, **P<0.01, and ***P<0.001). Data are expressed as mean±standard deviation (SD). Color images available online at www.liebertpub.com/scd
NRG-1β induces maturation of iPS-derived cardiac cells
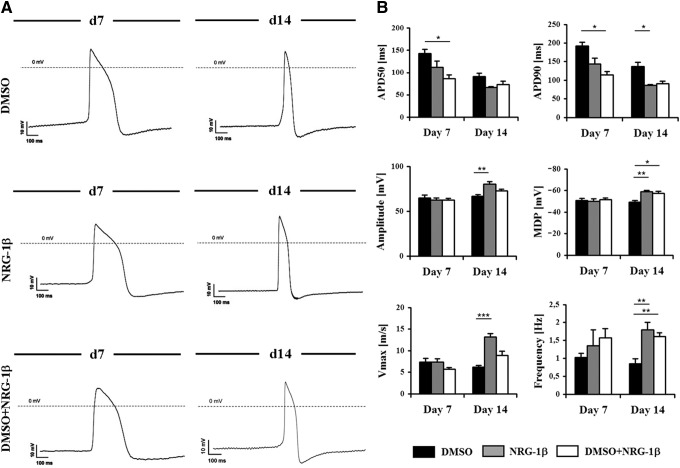
An improved cardiac differentiation was observed by the combined treatment with DMSO plus NRG-1β with the highest differences observed in differentially expressed cardiac gene induction and cardiac protein expression. However, differences in gene and even protein expression may not be reflected at the functional level. Thus, since functional properties of the cardiac cells are crucial for an efficient and safe regeneration, intracellular APs of iPS-CMs were evaluated by microelectrode recordings. The specific phenotype (atrial, ventricular, or nodal) and maturation state of CMs were analyzed at day 7 and 14 of differentiation in the three experimental groups (Fig. 3A). Intracellular AP recordings demonstrated the existence of viable iPS-CMs and revealed ventricular-like APs for all of the groups (Fig. 3A). Moreover, we did not identify atrial or pacemaker CMs, further suggesting specific differentiation toward a ventricular “working” phenotype. The following AP parameters were determined: AP duration at 50% repolarization (APD50), AP duration at 90% repolarization (APD90), amplitude, maximal diastolic potential (MDP), maximal upstroke velocity (Vmax), and frequency (Fig. 3B; Table 1). During native murine development, AP duration, and, in a greater manner, APD50 considerably decreases from embryonic to adult stage. Significantly lower values for APD50 and APD90 were detected at day 7 of differentiation in the group treated with DMSO+NRG-1β (P<0.05 vs. DMSO), indicating the formation of more mature CMs. A decrease in APD50 and APD90 values was also detected in the group treated with NRG-1β, although statistical significance was not reached. Interestingly, at day 14, the groups treated with NRG-1β (either with or without DMSO) showed lower APD50 and APD90 values being closer to values of NCMs (Table 1). Amplitude values for iPS-CMs increased from day 7 to 14 and were significantly higher in the groups treated with NRG-1β (P<0.01 vs. DMSO). These amplitudes were closer to those obtained with NCMs (Fig. 3B; Table 1). Moreover, MDP and Vmax values also increased by day 14 and were higher in the NRG-1β-treated groups (Table 1). Furthermore, frequency values were found to be significantly greater in the NRG-1β groups (compared with DMSO only; P<0.01; Table 1). In summary, CMs reached a more mature ventricular phenotype when treated with NRG-1β, showing higher MDP, amplitude, frequency, and Vmax values, as well as a shorter APD50 and APD90. In contrast, iPS-CMs differentiated in the presence of DMSO only also had a ventricular subtype differentiation but exhibited more immature properties, displaying low MDP, frequency, amplitude, and Vmax values, as well as a longer APD50 and APD90 (Table 1). Taken together, these findings support the role of NRG-1β in maturation of iPS-derived CMs.
FIG. 3.
Electrophysiological properties of iPS-CMs. (A) Phenotype of iPS-CMs. Cells were differentiated by treatment with DMSO and/or NRG-1β. The electrophysiological properties were analyzed at day 7 and 14 of differentiation. Spontaneous action potential (AP) recordings indicated that ventricular-like differentiation had occurred in all of the experimental groups. (B) Electrophysiological maturation of iPS-CMs. APD50, APD90, amplitude, maximal diastolic potential (MDP), Vmax, and frequency parameters were measured at day 7 and 14. Measurements (n=10–14) were performed in several beating clusters from each experimental group. Even though the AP parameters of iPS-CMs were consistent with cultivated fetal CMs, more mature electrophysiological properties were associated with NRG-1β-treated CMs. Results are shown as mean±SD. *P<0.05, **P<0.01, and ***P<0.001 in comparison with DMSO.
Table 1.
Action Potential Parameters
| Day | Cell type | APD50 (ms) | APD90 (ms) | Amplitude (mV) | MDP (mV) | Vmax (m/s) | Frequency (Hz) |
|---|---|---|---|---|---|---|---|
| Day 7 | DMSO | 143.2±9.3 | 192.1±10.3 | 64.8±3.4 | −50.8±1.9 | 7.3±0.8 | 1.0±0.1 |
| NRG-1β | 112.2±13.0 | 143.6±15.9 | 62.3±2.2 | −49.8±2.4 | 7.3±0.7 | 1.3±0.4 | |
| DMSO+NRG-1β | 86.3±8.5* | 114.2±9.0* | 62.4±1.9 | −51.4±1.6 | 5.6±0.4 | 1.5±0.2 | |
| Day 14 | DMSO | 91.4±6.9 | 136.9±11.7 | 66.8±1.6 | −49.1±1.4 | 6.1±0.4 | 0.8±0.1 |
| NRG-1β | 66.6±1.7 | 86.4±2.2* | 80.2±3.0** | −58.7±1.2** | 13.1±0.8*** | 1.7±0.2** | |
| DMSO+NRG-1β | 73.4±7.0 | 90.4±7.4 | 72.8±1.9 | −57.1±2.0* | 8.8±1.0 | 1.6±0.1** | |
| NCMs | 33.8±0.6 | 53.7±0.8 | 89.1±0.8 | −68.7±1.5 | 83.5±7.2 | 2.3±0.1 |
Results are shown as mean±standard deviation.
P<0.05, **P<0.01, ***P<0.001 in comparison with DMSO.
APD50, action potential duration at 50% repolarization; APD90, action potential duration at 90% repolarization; APD50/90, ratio APD50 versus APD90; MDP, maximal diastolic potential; Vmax, maximal upstroke velocity; NCMs, neonatal cardiomyocytes; DMSO, dimethyl sulfoxide; NRG-1β, neuregulin-1β.
Transplantation of iPS-CMs in a mouse model of AMI preserves cardiac function
CMs were generated from clone iPS-1 in the presence of NRG-1β and DMSO, which at day 7 induced a more highly differentiated and more mature phenotype than treatments alone. We transplanted iPS-CMs (2×105 on day 7 of differentiation) into the peri-infarct area of 8-week-old female DBA/2J mice (n=15) after permanent occlusion of the coronary artery. In addition, a control group (n=13) received media alone. Cardiac function was assessed by echocardiography at 2, 30, and 60 days after cell transplantation. We observed functional deterioration in the control group, with a significant decrease in the left ventricular ejection fraction and fractional area change at 60 days post-transplantation (compared with day 2 baseline). However, function was preserved in animals treated with iPS-CMs (Fig. 4 and Supplementary Table S1).
FIG. 4.
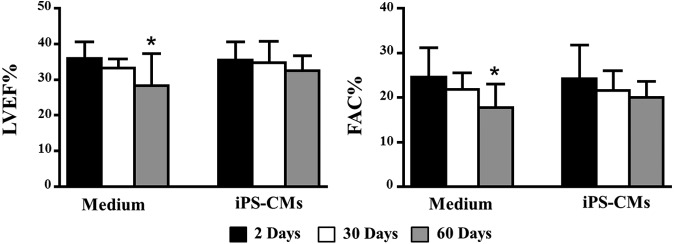
Transplantation of iPS-CMs preserves cardiac function. Cardiac function was measured by echocardiography at 2, 30, and 60 days post-transplantation in control (medium) and treated animals (iPS-CMs). Left ventricular ejection fraction percentage (LVEF%) and fractional area change percentage (FAC%) are displayed (*P<0.05, between 2 and 60 days). Data are expressed as mean±SD.
iPS-CMs engraft into host cardiac tissue and electromechanically couple with host CMs
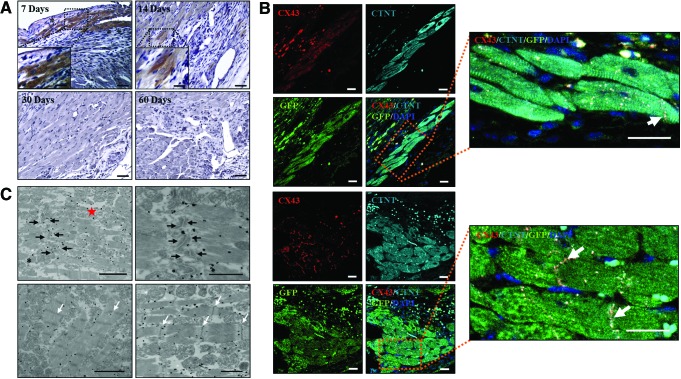
Engraftment of GFP+ (αMHC+) iPS-CMs could be detected by immunofluorescence at 7 and 14 days after transplantation [day 7: 3.8±4.6%; day 14: 0.3±0.4% (of total implanted cells)], whereas no GFP-positive signals were detected at longer time points [30 and 60 days after transplantation (Fig. 5A)]. In addition, GFP+ engrafted cells were found to co-express cardiac Troponin as well as Cx43, which suggested efficient electrical coupling of engrafted cells through gap junctions (Fig. 5B). Immunogold labeling for GFP and analysis via transmission electron microscopy (Fig. 5C) confirmed the presence of GFP+ iPS-CMs integrated into the heart tissue. We could observe organized sarcomeres with Z-lines and abundant mitochondria, a feature of high energy-consuming tissues. Intercalated discs composed of desmosomes were detected between GFP+ cells and host CMs, indicating the establishment of intercellular connections.
FIG. 5.
Fate of iPS-CMs after transplantation. (A) Analysis of cell engraftment. Representative areas of injured myocardium are shown at 7, 14, 30, and 60 days after transplantation. GFP+ areas were observed at day 7 and 14, but no GFP+ signals were detected at longer time points (day 30 and 60). (B) Transplanted CMs were GFP+ [αMHC; (green), cardiac Troponin+ (cTNT; cyan), and Cx43+ (red)]. Connexin 43 (Cx-43) protein was distributed between the CMs, indicating the presence of gap junctions (electrical coupling). Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). (C) Transmission electron microscopy of iPS-CMs at 7 days post-transplantation. Presence of GFP+ transplanted iPS-CMs was confirmed by electron microscopy (n=3). Organized and occasionally ramified myofibrils with Z-lines were detected throughout the cytoplasm (white arrows). Intercalated discs composed of desmosomes (black arrows) were detected between GFP+ iPS-CMs (red star), indicating mechanical coupling. Scale bars: (A) 50 μm; (B) 20 μm; (C) 2 μm (higher magnification: 1 μm). Color images available online at www.liebertpub.com/scd
Transplantation of iPS-CMs inhibits adverse cardiac remodeling and promotes revascularization of cardiac tissue
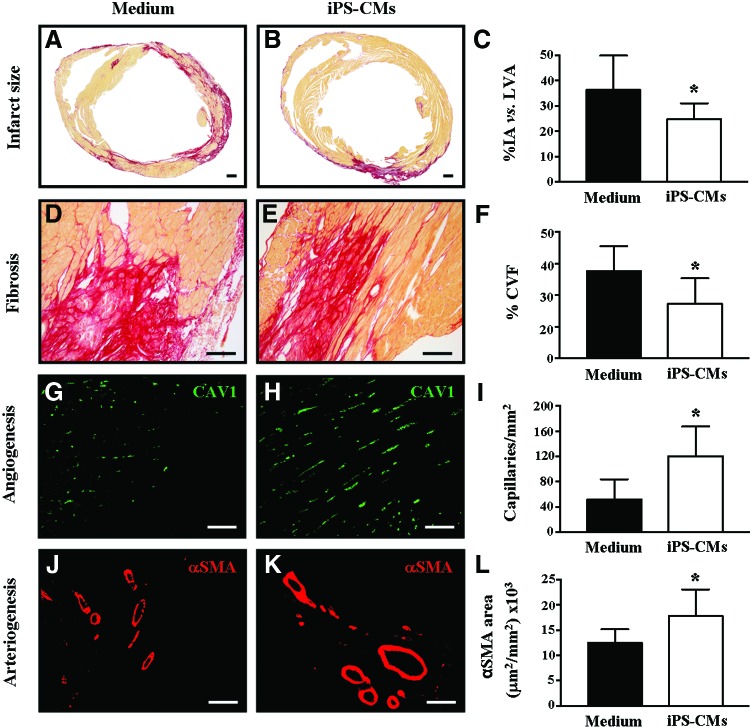
Lack of long-term engraftment of iPS-CMs suggested that the observed functional effects resulted from indirect mechanisms. Therefore, cardiac remodeling was examined next, and, indeed, transplantation of iPS-CMs led to a decrease in adverse cardiac remodeling with smaller infarct size (medium: 36.2±13.6%, iPS-CMs: 24.7±6.3%, P<0.05) (Fig. 6A–C) and reduced fibrosis (medium: 43.8±4.0%, iPS-CMs: 38.6±4.1%, P<0.05) (Fig. 6D–F) compared with the control. Finally, the angiogenic effect of transplantation of iPS-CMs was analyzed in the peri-infarcted area. A significant increase in tissue revascularization was detected at 2 months post-transplantation in the animals treated with iPS-CMs as compared with controls. This increase could be observed for both capillaries (small caliber vessels) (medium: 52±31 capillaries/mm2, iPS-CMs: 120±47 capillaries/mm2, P<0.05) (Fig. 6G–I) and larger vessels (arteries/arterioles) (medium: 12.5±2.6×103 μm2/mm2, iPS-CMs: 17.8±5.2×103 μm2/mm2, P<0.05) (Fig. 6J–L).
FIG. 6.
Transplantation of iPS-CMs is associated with adequate tissue remodeling. Morphometric analysis was analyzed at 2 months after transplantation of either medium or iPS-CMs. Serial Sirius-red staining of sections revealed significantly reduced scar sizes in the left ventricles of the iPS-CM-treated hearts compared with media-treated hearts (A–C). Data show the percentage of infarcted area (IA) versus the total left ventricular area (LVA). The degree of fibrosis was measured in the peri-infarct zone and was significantly lower with transplantation of iPS-CMs compared with the control (D–F). Capillary and arterioles/arteries densities were determined through quantification of caveolin-1+ (capillaries/mm2; 5–15 μm diameter) (G–I) and α-smooth muscle actin (SMA+) vessels (μm2/mm2) (J–L) in the peri-infarct area. Data are expressed as mean±SD (*P<0.05). Scale bars: (A, B) 500 μm; (D, E) 50 μm; (G, H, J, K) 100 μm. Color images available online at www.liebertpub.com/scd
Discussion
Although there has been great optimism surrounding the use of stem cells for cardiac regeneration, their clinical application has been limited by a failure to generate functional cardiac cells from adult stem cell populations as well as concerns over immunogenicity and tumorogenicity [1,10,29,30]. In the case of pluripotent stem cells, one attractive strategy for overcoming limitations involves in vitro differentiation before transplantation [8,31,32]. Nevertheless, several studies have indicated that most differentiation protocols result in CMs that lack a mature ventricular electrophysiological phenotype, despite the expression of cardiac markers [12]. Since NRG-1β is a key heart developmental factor (reviewed in [13]) that was recently shown to be involved in CM proliferation and differentiation [23,33,34], we explored its effect on iPS cell differentiation into CMs.
Strikingly, we found that in the presence of NRG-1β, murine iPS cells could be successfully differentiated into CMs. Furthermore, our expression array data demonstrated that the combined treatment of NRG-1β along with DMSO induced the most potent changes in the gene expression of cardiac-related genes, being closer to NCMs. The molecular mechanism by which NRG-1 stimulates cardiogenesis is still not clearly defined, although activation of PI3K/Akt and MEK-ERK signaling cascades as well as gene expression modulation through microRNAs (mir-296-p and mir-200c) has been shown [33–36]. Importantly, those iPS-CMs displayed a ventricular-like phenotype without differentiation into atrial or pacemaker CMs, suggesting the specificity of NRG-1β treatment. Confirming our results, previous studies with human ESC have also shown a modulation of nodal toward working-type cells after exogenous stimulus with NRG-1β [23]. This is a key point, as homogeneous ventricular differentiation is fundamental for transplantation, and because CMs with sustained pacemaker phenotypes might favor the development of cardiac arrhythmias [37,38]. Interestingly, the effects of NRG-1β were found to be synergistic with DMSO, a demethylating agent that was previously shown to induce cardiac differentiation in the P19 embryonic carcinoma cell line [39].
Notably, iPS-CMs that were differentiated in the presence of DMSO and NRG-1β exhibited properties which were more consistent with an electrophysiological maturation of CMs [40]. Recently, NRG-1β was used to induce differentiation of ESCs into CMs; however, it was found that even though the ESC-derived CMs responded to β-adrenergic and muscarinic agonists, they were less mature and had higher APD90 values (∼200 ms) [23] than what we observed in this study (∼86 ms). This discrepancy could be explained by differences in the in vitro protocols or between the ESC and iPS cell lines used.
We found that transplantation of iPS-CMs was associated with a functional benefit. In fact, we observed long-term preservation of cardiac function, smaller infarct size, reduced scaring, and induction of tissue revascularization. All these changes are consistent with other reports that have demonstrated positive remodeling after transplantation of human iPS-CMs [41]. Interestingly, no teratoma formation was observed after long-term follow up in any of the animals transplanted with iPS-CMs, suggesting that transplantation of differentiated cells can prevent iPS cell-mediated tumorigenesis [10].
Although iPS-CMs exhibited a more mature cardiac phenotype after 14 days, we decided to transplant 7-day iPS-CMs based on previous studies, which have indicated that fetal CMs more efficiently integrate and survive into the myocardium than primary adult CMs, perhaps partly resulting from their greater proliferation capacity and resistance to hypoxia [42,43]. In our study, engrafted cardiac cells were detected for approximately 2 weeks, displaying characteristic sarcomeric organization with Z-lines, aligned and ramified myofibrils, and abundant large mitochondria. Interestingly, intercalated discs composed of desmosomes were found to connect transplanted CMs with neighboring CMs (both host and transplanted), further suggesting that pre-differentiated CMs derived from iPS cells were capable of efficiently maturing in vivo. In addition, Cx43 expression was detected in the transplanted cells, consistent with electrical coupling among CMs through gap junctions. In comparison with our results, Mauritz et al. recently reported that iPS-derived Flk1+ cardiovascular progenitors were capable of engrafting into heart tissue; however, structured sarcomeres were not observed, indicating a more immature cardiac phenotype [44]. Moreover, recent studies have shown electromechanical coupling of mouse iPS cells and human ESC-derived CMs in mouse [45] and pig models [46], respectively, supporting the capacity of transplanted cells to contribute to the heart muscle. Nevertheless, despite these encouraging “proof-of-concept” results, there was only temporary or low-degree CM engraftment observed in these studies. This seems to indicate that the functional benefits associated with transplantation of iPS-CMs do not result mainly from direct contributions, but rather from indirect or trophic effects of iPS-CMs. This phenomena has been demonstrated for many (stem) cell types (reviewed in [47]), including adult CMs, which in response to hypoxia [48] and mechanical stress [49] release many factors that promote angiogenesis, cell survival, and CM regulation [50].
Indeed, the limited in vivo engraftment of stem and progenitor cells remains a major limitation for regenerative cardiac therapies. In this regard, several strategies are being tested to enhance stem cell survival and engraftment, such as combination approaches involving other cell populations (eg, vascular [51] or mesenchymal cell types [52]) and/or the use of bioengineered scaffolds [53,54]. Furthermore, sustained release of NRG-1β protein by controlled delivery systems could be an innovative therapy, potentiating the in vivo differentiation and maturation of the iPS-CMs as well as endogenous cardiac stem cells [55].
In summary, our results demonstrate that transplantation of iPS-CMs provides a long-term beneficial effect, preserving cardiac function and tissue viability in a mouse model of AMI. Implanted in an ischemic environment, these cells survive and electromechanically couple to cardiac tissue, inducing positive remodeling and promoting tissue revascularization. Furthermore, we have demonstrated that NRG-1β in combination with DMSO induces a homogenous and consistent differentiation of iPS cells toward a more mature ventricular-like cardiac phenotype. Thus, this method could be used to generate sufficient numbers of CMs with a higher degree of maturation for cellular transplantation. Taken together, this approach can be combined with newly developed strategies that enhance engraftment and survival in order to overcome limitations related to cardiac regenerative therapies.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. J.L. Field (Indianapolis University, Indiana, United States) for αMHC-GFP mice, Dr. Spits (Academic Medical Center of Amsterdam, Netherlands) for Rag2−/−γc−/− mice, and Dr. Claycomb (Louisiana State University Medical Center) for HL-1 cell line donation. They also thank the Unit of Proteomics, Genomics, and Bioinformatics of the Center for Applied Medical Research (CIMA). This work was supported in part by funds from the ISCIII (RD12/0036/0068, PI13/02144, PI10/01621, and CP09/00333), MINECO (PLE2009-0116, DPI2012-38090-C03-02, and INNPACTO Procardio); funding from the European Union Seventh Framework Program (FP7/2007-2013) (INELPY), Caja de Ahorros de Navarra (Programa Tu Eliges: Tu Decides), and the “UTE project CIMA.”
Author Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1.Passier R, van Laake LW. and Mummery CL. (2008). Stem-cell-based therapy and lessons from the heart. Nature 453:322–329 [DOI] [PubMed] [Google Scholar]
- 2.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, et al. (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776 [DOI] [PubMed] [Google Scholar]
- 3.Winter EM, van Oorschot AA, Hogers B, van der Graaf LM, Doevendans PA, Poelmann RE, Atsma DE, Gittenberger-de Groot AC. and Goumans MJ. (2009). A new direction for cardiac regeneration therapy: application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ Heart Fail 2:643–653 [DOI] [PubMed] [Google Scholar]
- 4.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, et al. (2007). Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res 1:9–24 [DOI] [PubMed] [Google Scholar]
- 5.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, et al. (2007). Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25:1015–1024 [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K. and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- 7.Iglesias-Garcia O, Pelacho B. and Prosper F. (2013). Induced pluripotent stem cells as a new strategy for cardiac regeneration and disease modeling. J Mol Cell Cardiol 62:43–50 [DOI] [PubMed] [Google Scholar]
- 8.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, et al. (2008). Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation 118:507–517 [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA. and Kamp TJ. (2009). Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res 104:e30–e41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y. and Terzic A. (2009). Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 120:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, et al. (2012). Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126:S29–S37 [DOI] [PubMed] [Google Scholar]
- 12.Xi J, Khalil M, Shishechian N, Hannes T, Pfannkuche K, Liang H, Fatima A, Haustein M, Suhr F, et al. (2010). Comparison of contractile behavior of native murine ventricular tissue and cardiomyocytes derived from embryonic or induced pluripotent stem cells. FASEB J 24:2739–2751 [DOI] [PubMed] [Google Scholar]
- 13.Odiete O, Hill MF. and Sawyer DB. (2012). Neuregulin in cardiovascular development and disease. Circ Res 111:1376–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R. and Sawyer DB. (2004). Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem 279:51141–51147 [DOI] [PubMed] [Google Scholar]
- 15.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA. and Kelly RA. (1998). Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem 273:10261–10269 [DOI] [PubMed] [Google Scholar]
- 16.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA. and Sawyer DB. (2003). Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol 35:1473–1479 [DOI] [PubMed] [Google Scholar]
- 17.Seguchi O, Takashima S, Yamazaki S, Asakura M, Asano Y, Shintani Y, Wakeno M, Minamino T, Kondo H, et al. (2007). A cardiac myosin light chain kinase regulates sarcomere assembly in the vertebrate heart. J Clin Invest 117:2812–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iivanainen E, Paatero I, Heikkinen SM, Junttila TT, Cao R, Klint P, Jaakkola PM, Cao Y. and Elenius K. (2007). Intra- and extracellular signaling by endothelial neuregulin-1. Exp Cell Res 313:2896–2909 [DOI] [PubMed] [Google Scholar]
- 19.Xu G, Watanabe T, Iso Y, Koba S, Sakai T, Nagashima M, Arita S, Hongo S, Ota H, et al. (2009). Preventive effects of heregulin-beta1 on macrophage foam cell formation and atherosclerosis. Circ Res 105:500–510 [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Gu X, Li Z, Li X, Li H, Chang J, Chen P, Jin J, Xi B, et al. (2006). Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol 48:1438–1447 [DOI] [PubMed] [Google Scholar]
- 21.Gu X, Liu X, Xu D, Li X, Yan M, Qi Y, Yan W, Wang W, Pan J, et al. (2010). Cardiac functional improvement in rats with myocardial infarction by up-regulating cardiac myosin light chain kinase with neuregulin. Cardiovasc Res 88:334–343 [DOI] [PubMed] [Google Scholar]
- 22.Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE. and Fishman GI. (2002). Neuregulin-1 promotes formation of the murine cardiac conduction system. Proc Natl Acad Sci U S A 99:10464–10469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B. and Laflamme MA. (2010). Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res 107:776–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B. and Speed TP. (2003). Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentleman R, Carey V, Dudoit S, Irizarry R. and Huber W. (2005). Bioinformatics and Computational Biology Solutions Using R and Bioconductor. Springer, NY [Google Scholar]
- 26.Gene Ontology Consortium, Blake JA, Dolan M, Drabkin H, Hill DP, Li N, Sitnikov D, Bridges S, Burgess S, et al. (2013). Gene Ontology annotations and resources. Nucleic Acids Res 41:D530–D535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelacho B, Nakamura Y, Zhang J, Ross J, Heremans Y, Nelson-Holte M, Lemke B, Hagenbrock J, Jiang Y, et al. (2007). Multipotent adult progenitor cell transplantation increases vascularity and improves left ventricular function after myocardial infarction. J Tissue Eng Regen Med 1:51–59 [DOI] [PubMed] [Google Scholar]
- 28.Benavides-Vallve C, Corbacho D, Iglesias-Garcia O, Pelacho B, Albiasu E, Castano S, Munoz-Barrutia A, Prosper F. and Ortiz-de-Solorzano C. (2012). New strategies for echocardiographic evaluation of left ventricular function in a mouse model of long-term myocardial infarction. PLoS One 7:e41691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behfar A, Perez-Terzic C, Faustino RS, Arrell DK, Hodgson DM, Yamada S, Puceat M, Niederlander N, Alekseev AE, Zingman LV. and Terzic A. (2007). Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med 204:405–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada S, Nelson T, Kane G, Martinez-Fernandez A, Crespo-Diaz RJ, Ikeda Y, Terzic C. and Terzic A. (2013). iPS cell intervention rescues wall motion disparity achieving biological cardiac resynchronization post-infarction. J Physiol 591:4335–4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zwi L, Caspi O, Arbel G, Huber I, Gepstein A, Park IH. and Gepstein L. (2009). Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation 120:1513–1523 [DOI] [PubMed] [Google Scholar]
- 32.Uosaki H, Fukushima H, Takeuchi A, Matsuoka S, Nakatsuji N, Yamanaka S. and Yamashita JK. (2011). Efficient and scalable purification of cardiomyocytes from human embryonic and induced pluripotent stem cells by VCAM1 surface expression. PLoS One 6:e23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Xu G, Wu Y, Guan Y, Cui L, Lei X, Zhang J, Mou L, Sun B. and Dai Q. (2009). Neuregulin-1 enhances differentiation of cardiomyocytes from embryonic stem cells. Med Biol Eng Comput 47:41–48 [DOI] [PubMed] [Google Scholar]
- 34.Sun M, Yan X, Bian Y, Caggiano AO. and Morgan JP. (2011). Improving murine embryonic stem cell differentiation into cardiomyocytes with neuregulin-1: differential expression of microRNA. Am J Physiol Cell Physiol 301:C21–C30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HS, Cho JW, Hidaka K. and Morisaki T. (2007). Activation of MEK-ERK by heregulin-beta1 promotes the development of cardiomyocytes derived from ES cells. Biochem Biophys Res Commun 361:732–738 [DOI] [PubMed] [Google Scholar]
- 36.Hao J, Galindo CL, Tran TL. and Sawyer DB. (2014). Neuregulin-1beta induces embryonic stem cell cardiomyogenesis via ErbB3/ErbB2 receptors. Biochem J 458:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang YM, Hartzell C, Narlow and M.Dudley SC, Jr., (2002). Stem cell-derived cardiomyocytes demonstrate arrhythmic potential. Circulation 106:1294–1299 [DOI] [PubMed] [Google Scholar]
- 38.Chen HS, Kim C. and Mercola M. (2009). Electrophysiological challenges of cell-based myocardial repair. Circulation 120:2496–2508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards MK, Harris JF. and McBurney MW. (1983). Induced muscle differentiation in an embryonal carcinoma cell line. Mol Cell Biol 3:2280–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halbach M, Pfannkuche K, Pillekamp F, Ziomka A, Hannes T, Reppel M, Hescheler J. and Muller-Ehmsen J. (2007). Electrophysiological maturation and integration of murine fetal cardiomyocytes after transplantation. Circ Res 101:484–492 [DOI] [PubMed] [Google Scholar]
- 41.Carpenter L, Carr C, Yang CT, Stuckey DJ, Clarke K. and Watt SM. (2011). Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells Dev 21:977–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinecke H, Zhang M, Bartosek T. and Murry CE. (1999). Survival, integration, and differentiation of cardiomyocyte grafts: a study in normal and injured rat hearts. Circulation 100:193–202 [DOI] [PubMed] [Google Scholar]
- 43.Halbach M, Baumgartner S, Sahito RG, Krausgrill B, Maass M, Peinkofer G, Ladage D, Hescheler J. and Muller-Ehmsen J. (2014). Cell persistence and electrical integration of transplanted fetal cardiomyocytes from different developmental stages. Int J Cardiol 171:e122–e124 [DOI] [PubMed] [Google Scholar]
- 44.Mauritz C, Martens A, Rojas SV, Schnick T, Rathert C, Schecker N, Menke S, Glage S, Zweigerdt R, et al. (2011). Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur Heart J 32:2634–2641 [DOI] [PubMed] [Google Scholar]
- 45.Halbach M, Peinkofer G, Baumgartner S, Maass M, Wiedey M, Neef K, Krausgrill B, Ladage D, Fatima A, et al. (2013). Electrophysiological integration and action potential properties of transplanted cardiomyocytes derived from induced pluripotent stem cells. Cardiovasc Res 100:432–440 [DOI] [PubMed] [Google Scholar]
- 46.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, et al. (2012). Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489:322–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M. and Dzau VJ. (2010). Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol 50:280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki H, Ray PS, Zhu L, Galang N. and Maulik N. (2000). Oxidative stress due to hypoxia/reoxygenation induces angiogenic factor VEGF in adult rat myocardium: possible role of NFkappaB. Toxicology 155:27–35 [DOI] [PubMed] [Google Scholar]
- 49.Seko Y, Takahashi N, Tobe K, Kadowaki T. and Yazaki Y. (1999). Pulsatile stretch activates mitogen-activated protein kinase (MAPK) family members and focal adhesion kinase (p125(FAK)) in cultured rat cardiac myocytes. Biochem Biophys Res Commun 259:8–14 [DOI] [PubMed] [Google Scholar]
- 50.Zentilin L, Puligadda U, Lionetti V, Zacchigna S, Collesi C, Pattarini L, Ruozi G, Camporesi S, Sinagra G, et al. (2009). Cardiomyocyte VEGFR-1 activation by VEGF-B induces compensatory hypertrophy and preserves cardiac function after myocardial infarction. FASEB J 24:1467–1478 [DOI] [PubMed] [Google Scholar]
- 51.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H. and Murry CE. (2009). Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A 106:16568–16573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Templin C, Zweigerdt R, Schwanke K, Olmer R, Ghadri JR, Emmert MY, Muller E, Kuest SM, Cohrs S, et al. (2012). Transplantation and tracking of human-induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation 126:430–439 [DOI] [PubMed] [Google Scholar]
- 53.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H. and Murry CE. (2011). Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res 109:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiong Q, Hill KL, Li Q, Suntharalingam P, Mansoor A, Wang X, Jameel MN, Zhang P, Swingen C, Kaufman DS. and Zhang J. (2011). A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells 29:367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Formiga FR, Pelacho B, Garbayo E, Imbuluzqueta I, Diaz-Herraez P, Abizanda G, Gavira JJ, Simon-Yarza T, Albiasu E, et al. (2014). Controlled delivery of fibroblast growth factor-1 and neuregulin-1 from biodegradable microparticles promotes cardiac repair in a rat myocardial infarction model through activation of endogenous regeneration. J Control Release 173:132–139 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.