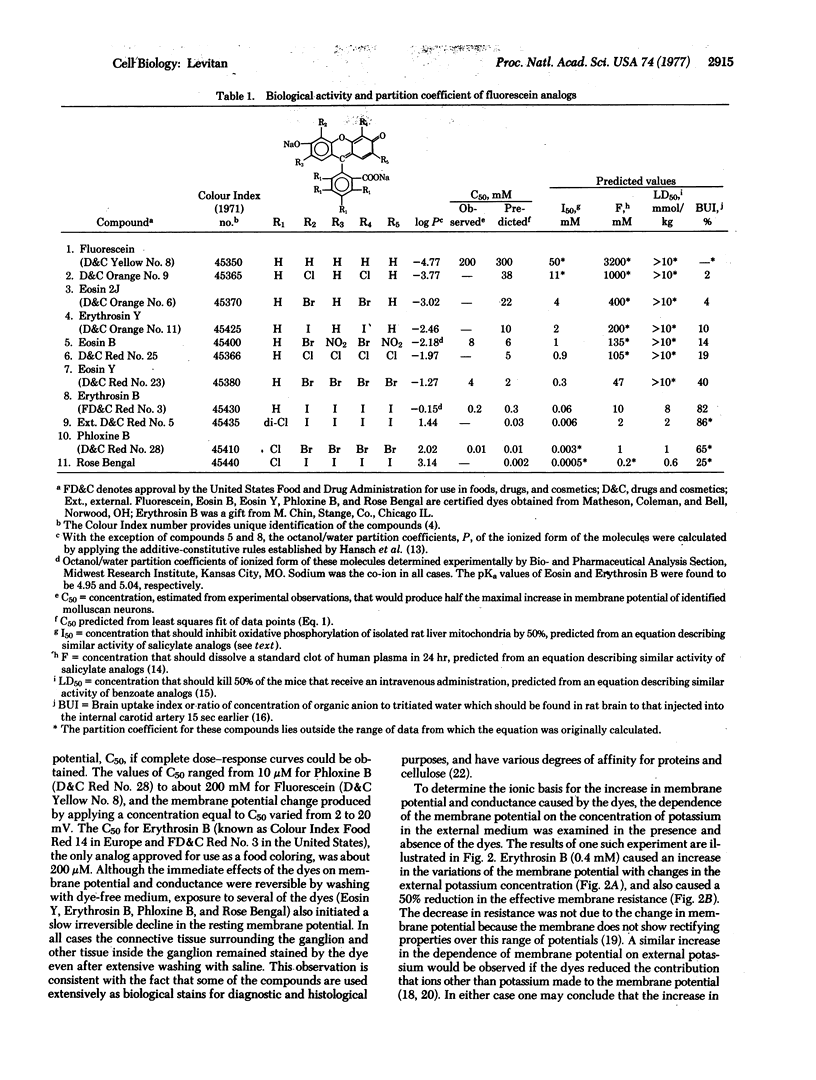
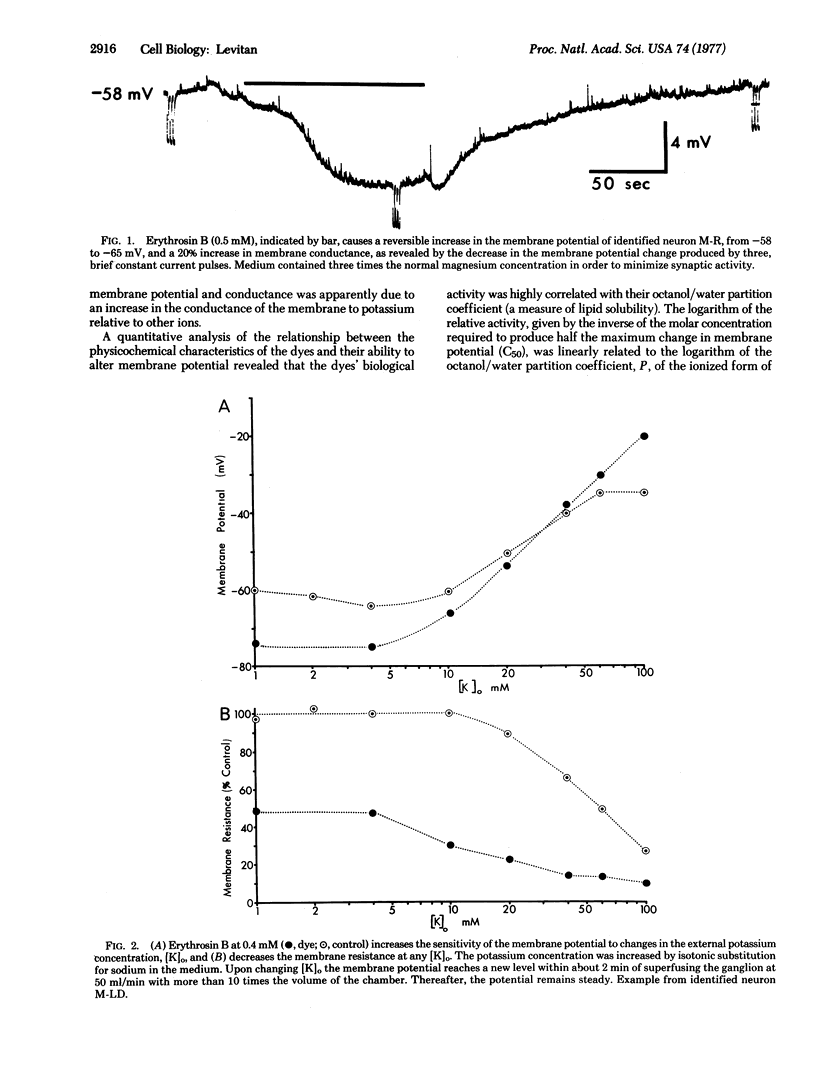
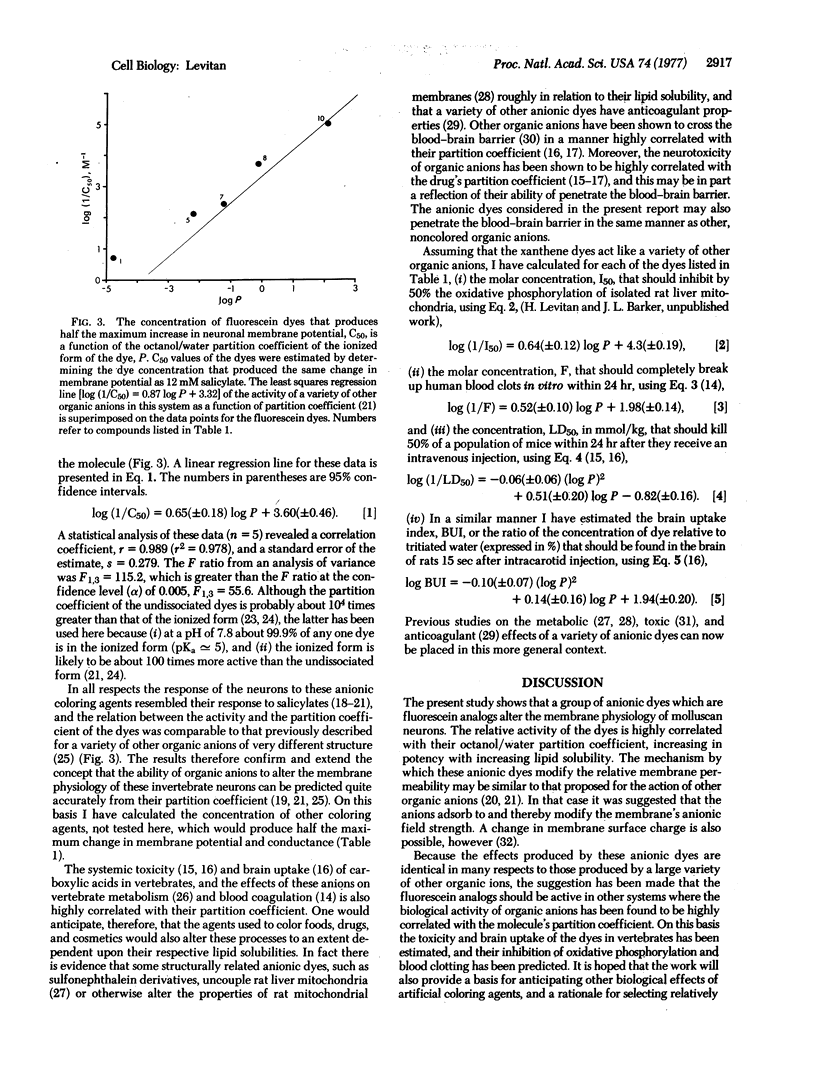
Abstract
Food, drug, and cosmetic dyes of the xanthane type (analogs of fluorescein) were applied to isolated molluscan ganglia and changes in the electrophysiological properties of identified neurons were monitored. The synthetic coloring agents increased the resting membrane potential and conductance of the neurons in a dose-dependent manner by increasing the potassium permeability of the membrane relative to that of other ions. The relative activity of these anionic dyes was highly correlated with their lipid solubility. The structure-activity study of the effects of the dyes on molluscan neurophysiology provides a basis for estimating the toxicity and brain uptake of the dyes in vertebrates, and predicting their effects on metabolism and blood clotting.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksandrowicz Z., Sweirczyński J. The inhibition by bromothymol blue of anion translocation across the mitochondrial membrane. Biochim Biophys Acta. 1975 Feb 28;382(1):92–105. doi: 10.1016/0005-2736(75)90376-4. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Levitan H. Acetanilides: effects on invertebrate neurons correlated with analgesic activity in vertebrates. J Pharmacol Exp Ther. 1975 Jun;193(3):892–902. [PubMed] [Google Scholar]
- Barker J. L., Levitan H. Mitochondrial uncoupling agents. Effects on membrane permeability of molluscan neurons. J Membr Biol. 1975;25(3-4):361–380. doi: 10.1007/BF01868584. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Levitan H. Salicylate: effect on membrane permeability of molluscan neurons. Science. 1971 Jun 18;172(3989):1245–1247. doi: 10.1126/science.172.3989.1245. [DOI] [PubMed] [Google Scholar]
- Boffey P. M. Color additives: botched experiment leads to banning of red dye no. 2. Science. 1976 Feb 6;191(4226):450–451. doi: 10.1126/science.191.4226.450. [DOI] [PubMed] [Google Scholar]
- Drake J. J. Food colours--harmless aesthetics or epicurean luxuries? Toxicology. 1975 Sep;5(1):3–42. doi: 10.1016/0300-483x(75)90067-0. [DOI] [PubMed] [Google Scholar]
- Feingold B. F. Hyperkinesis and learning disabilities linked to artificial food flavors and colors. Am J Nurs. 1975 May;75(5):797–803. [PubMed] [Google Scholar]
- Hansch C., Glave W. R. Structure-activity relationships in membrane-perturbing agents. Hemolytic, narcotic, and antibacterial compounds. Mol Pharmacol. 1971 May;7(3):337–354. [PubMed] [Google Scholar]
- Hansch C., Leo A., Unger S. H., Kim K. H., Nikaitani D., Lien E. J. "Aromatic" substituent constants for structure-activity correlations. J Med Chem. 1973 Nov;16(11):1207–1216. doi: 10.1021/jm00269a003. [DOI] [PubMed] [Google Scholar]
- Huggett A. S., Rowe F. M. The relationship of azo dyes to the coagulation of blood. J Physiol. 1933 Nov 9;80(1):82–95. doi: 10.1113/jphysiol.1933.sp003073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killenberg P. G., Hoppel C. L. Inhibition of rat liver mitochondrial oxidative phosphorylation by sulfobromophthalein. Mol Pharmacol. 1974 Jan;10(1):108–118. [PubMed] [Google Scholar]
- Levitan H., Barker J. L. Effect of non-narcotic analgesics on membrane permeability of molluscan neurones. Nat New Biol. 1972 Sep 13;239(89):55–57. doi: 10.1038/newbio239055a0. [DOI] [PubMed] [Google Scholar]
- Levitan H., Barker J. L. Membrane permeability: cation selectivity reversibly altered by salicylate. Science. 1972 Oct 6;178(4056):63–64. doi: 10.1126/science.178.4056.63. [DOI] [PubMed] [Google Scholar]
- Levitan H., Barker J. L. Salicylate: a structure-activity study of its effects on membrane permeability. Science. 1972 Jun 30;176(4042):1423–1425. doi: 10.1126/science.176.4042.1423. [DOI] [PubMed] [Google Scholar]
- Levitan H., Rapoport S. I. Contrast media: quantitative criteria for designing compounds with low toxicity. Acta Radiol Diagn (Stockh) 1976 Jan;17(1):81–92. doi: 10.1177/028418517601700107. [DOI] [PubMed] [Google Scholar]
- Levitan H., Tauc L., Segundo J. P. Electrical transmission among neurons in the buccal ganglion of a mollusc, Navanax inermis. J Gen Physiol. 1970 Apr;55(4):484–496. doi: 10.1085/jgp.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. The mechanism of action of DNP on phospholipid bilayer membranes. J Membr Biol. 1972;9(4):361–372. [PubMed] [Google Scholar]
- Oldendorf W. H. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am J Physiol. 1973 Jun;224(6):1450–1453. doi: 10.1152/ajplegacy.1973.224.6.1450. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I., Levitan H. Neurotoxicity of X-ray contrast media. Relation to lipid solubility and blood-brain barrier permeability. Am J Roentgenol Radium Ther Nucl Med. 1974 Sep;122(1):186–193. doi: 10.2214/ajr.122.1.186. [DOI] [PubMed] [Google Scholar]
- WHITEHOUSE M. W. BIOCHEMICAL PROPERTIES OF ANTI-INFLAMMATORY DRUGS--III. UNCOUPLING OF OXIDATIVE PHOSPHORYLATION IN A CONNECTIVE TISSUE (CARTILAGE) AND LIVER MITOCHONDRIA BY SALICYLATE ANALOGUES: RELATIONSHIP OF STRUCTURE TO ACTIVITY. Biochem Pharmacol. 1964 Mar;13:319–336. doi: 10.1016/0006-2952(64)90148-0. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M., von Kaulla K. N., Hansch C. Structure-activity relationship in synthetic fibrinolytics. 2-Phenethynylcyclopropanecarboxylates. J Med Chem. 1975 Sep;18(9):950–951. doi: 10.1021/jm00243a019. [DOI] [PubMed] [Google Scholar]


