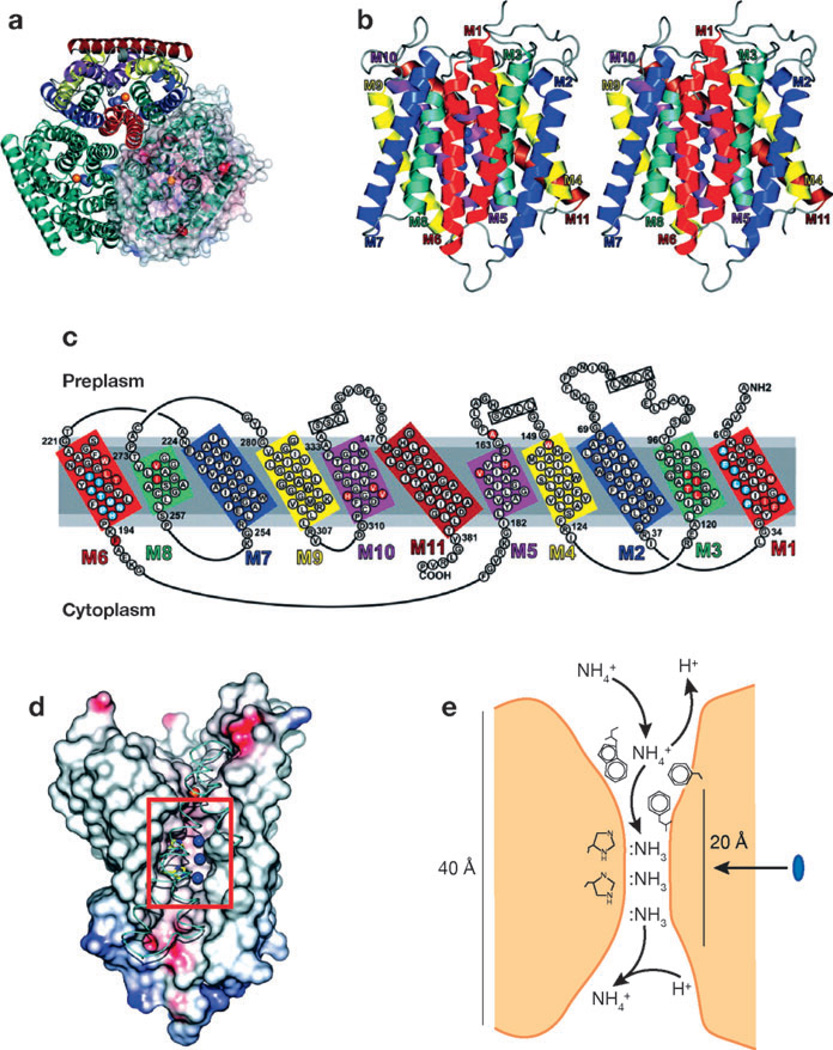
Figure 4.
Tertiary structure of E. coli AmtB. (a) A ribbon representation of AmtB viewed from the extracellular surface demonstrates the trimeric structure of AmtB. In the top monomer, homologous transmembrane segments are shown in the same color. In the right monomer, a solvent-accessible transparent surface is colored according to the electrostatic potential (red for negative and blue for positive). Potential ammonia-binding sites are shown as the blue sphere for NH3 and red sphere for NH4+. (b) A stereoview of the monomeric structure. The extracellular surface is uppermost. NH3-binding sites are shown as blue spheres. (c) The amino acid sequences of the transmembrane extracellular and intracellular loops. Homologous sequences are shown in similar colors. (d) The ammonia-conducting portion of AmtB after removal of portions of helices M8, M9, and M10. The lumen surface is colored according to the electrostatic potential (red for negative and blue for positive). The histidines near the three NH3 sites (blue spheres) are shown in yellow. Narrow hydrophobic regions through the conducting channel lie above and below the NH3 sites. (e) The deduced mechanism of ammonia transport through AmtB. The blue oval indicates the 20-Å hydrophobic channel of the protein. Figure from Reference 116, with permission from AAAS.