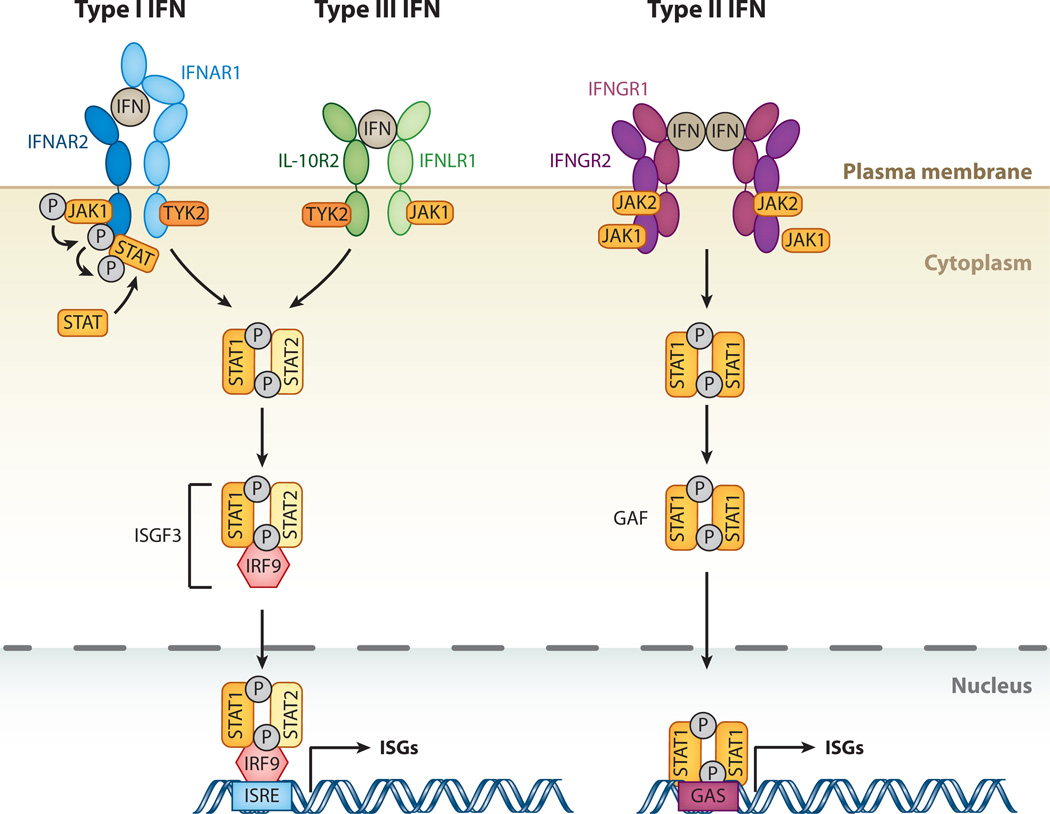
Figure 1.
The interferon (IFN)-signaling cascade. The three different classes of IFNs signal through distinct receptor complexes on the cell surface: type I IFNs act through IFN-α receptor 1 (IFNAR1) and 2 (IFNAR2) heterodimers; type III IFN through interleukin-10 receptor 2 (IL-10R2) and IFN-λ receptor 1 (IFNLR1) heterodimers; and type II IFN through dimers of heterodimers consisting of IFN-γ receptors 1 (IFNGR1) and 2 (IFNGR2). Binding of both type I and type III IFNs to their IFNAR1/2 or IL-10R2/IFNLR1 complexes, respectively, triggers phosphorylation of preassociated Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2), which in turn phosphorylate the receptors at specific intracellular tyrosine residues. This leads to the recruitment and phosphorylation of signal transducers and activators of transcription 1 and 2 (STAT1 and 2). STAT1 and 2 associate to form a heterodimer, which in turn recruits the IFN-regulatory factor 9 (IRF9) to form the IFN-stimulated gene factor 3 (ISGF3). Binding of type II IFN dimers to the IFNGR1/2 complex leads to phosphorylation of preassociated JAK1 and JAK2 tyrosine kinases, and transphosphorylation of the receptor chains leads to recruitment and phosphorylation of STAT1. Phosphorylated STAT1 homodimers form the IFN-γ activation factor (GAF). Both ISGF3 and GAF translocate to the nucleus to induce genes regulated by IFN-stimulated response elements (ISRE) and gamma-activated sequence (GAS) promoter elements, respectively, resulting in expression of antiviral genes.