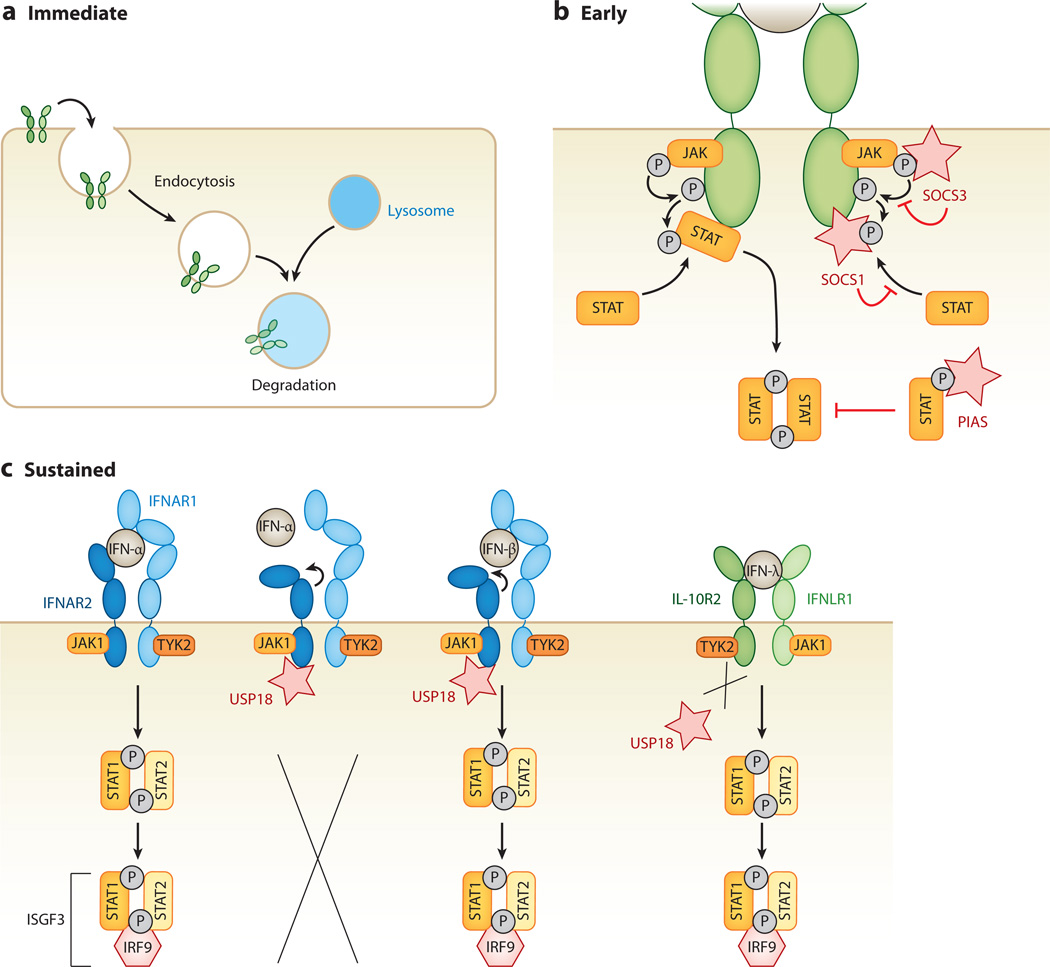
Figure 3.
Interferon (IFN) desensitization pathways. IFN signaling is negatively regulated by various mechanisms. (a) An immediate mechanism of IFN desensitization is endocytosis and turnover of IFN receptors, which rapidly reduces the level of JAK-STAT signaling within the cell. (b) Another early mechanism of IFN desensitization requires de novo synthesis of inhibitory proteins. IFN-stimulated suppressor of cytokine signaling (SOCS) proteins act as kinase inhibitors within the JAK-STAT phosphorylation cascade. Both SOCS1 and SOCS3 act as pseudosubstrates for receptor-associated JAKs. Protein inhibitors of activated STAT (PIAS) proteins bind to and inhibit phosphorylated STATs, thereby interrupting the signaling cascade. (c) The ubiquitin-specific peptidase 18 (USP18), expressed from an IFN-stimulated gene, leads to a more sustained shutdown of JAK-STAT signaling. USP18 binds to the intracellular side of the IFN-α receptor 2 (IFNAR2), resulting in conformational changes in the extracellular domains of IFNAR2, which keeps low-affinity IFNs such as IFN-α from binding, and hence from inducing, the JAK-STAT signaling cascade. In contrast, IFNs with higher receptor affinity, such as IFN-β, are still able to bind and initiate the signaling cascade. USP18 binding is specific to IFNAR2, and thus it does not interfere with type II or type III IFN signaling.