Abstract
Background
Prevalence of chronic hepatitis C virus (HCV) infection is high among incarcerated persons in the United States. New, short-duration, high-efficacy therapies may expand treatment eligibility in this population.
Objective
To assess the cost-effectiveness of sofosbuvir for HCV treatment in incarcerated populations.
Design
Markov model.
Data Sources
Published literature and expert opinion.
Target Population
Treatment-naive men with chronic, genotype 1 HCV monoinfection.
Time Horizon
Lifetime.
Perspective
Societal.
Intervention
No treatment, 2-drug therapy (pegylated interferon and ribavirin), or 3-drug therapy with either boceprevir or sofosbuvir. For inmates with short remaining sentences (<1.5 years), only no treatment or sofosbuvir 3-drug therapy were feasible; for those with long sentences (≥1.5 years; mean, 10 years), all strategies were considered. After release, eligible persons could receive sofosbuvir 3-drug therapy.
Outcome Measures
Discounted costs (in 2013 U.S. dollars), discounted quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios.
Results of Base-Case Analysis
The strategies yielded 13.12, 13.57, 14.43, and 15.18 QALYs, respectively, for persons with long sentences. Sofosbuvir produced the largest absolute reductions in decompensated cirrhosis (16%) and hepatocellular carcinoma (9%), resulting in 2.1 additional QALYs at an added cost exceeding $54 000 compared with no treatment. For persons with short sentences, sofosbuvir cost $25 700 per QALY gained compared with no treatment; for those with long sentences, it dominated other treatments, costing $28 800 per QALY gained compared with no treatment.
Results of Sensitivity Analysis
High reinfection rates in prison attenuated cost-effectiveness for persons with long sentences.
Limitations
Data on sofosbuvir’s long-term effectiveness and price are limited. The analysis did not consider women, Hispanic persons, or patients co-infected with HIV or hepatitis B virus.
Conclusion
Sofosbuvir-based treatment is cost-effective for incarcerated persons, but affordability is an important consideration.
Primary Funding Source
National Institutes of Health.
In the United States, more than 500 000 incarcerated persons have chronic hepatitis C virus (HCV) infection (1–3). Chronic HCV infection causes liver fibrosis, cirrhosis, hepatocellular carcinoma, and the need for liver transplant (4). The recent availability of short-duration, highly efficacious treatments (5–10) may be advantageous for patients in this population given that they are less likely to be treated after being released. Targeting chronic HCV infection in prisons, where the prevalence is 12% to 35% (nearly 10 times the overall U.S. prevalence), represents a public health opportunity (3, 11).
Correctional systems lack a common HCV protocol. In 2000, 76% of U.S. adult correctional facilities tested inmates for HCV and 70% reported a treatment policy (12). Recent data suggest increases in testing, although many diagnosed inmates remain untreated (13–15). Treatment initiation rules vary but often require remaining sentences of more than 18 to 24 months to enable completion before release (15). Evidence from other populations (16) and new short-duration treatments may obviate these rules provided that treatment is delivered cost-effectively.
Treatment of HCV in correctional facilities is challenging. Unplanned transfers and releases can disrupt treatment and may select for viral resistance (15). Higher reinfection risks after cure can reduce treatment benefits for incarcerated persons. High costs of administering directly acting antivirals represent a formidable barrier (14).
Depending on their costs, directly acting antivirals may shift the balance toward treatment expansion. Until recently, standard-of-care treatment was 2-drug therapy with pegylated interferon and ribavirin. Despite 48 weeks of treatment, sustained virologic response (SVR) rates can be as low as 45% for genotype 1 HCV (4) and even lower in black patients, who are overrepresented in incarcerated populations (17, 18). Since 2011, the U.S. Food and Drug Administration (FDA) has approved 4 directly acting antivirals with SVR rates exceeding 75% to 90% in trials: the protease inhibitors boceprevir, telaprevir, and simeprevir and the polymerase inhibitor sofosbuvir, each used in combination with interferon and ribavirin (7, 19). Newer, all-oral, interferon-sparing regimens have shown high efficacy but are not yet FDA-approved (8, 10). New FDA-approved regimens have durations as short as 12 weeks (sofosbuvir) (20), but costs exceed $7000 per week (21).
We built on previous analyses (22–28) by evaluating the cost-effectiveness of expanding HCV treatment to incarcerated persons, including those with short remaining sentences.
Methods
Overview
We used a decision analytic Markov model (24, 29, 30) to follow cohorts of treatment-naive, incarcerated men with chronic, genotype 1 HCV monoinfection. The cohorts were stratified by liver fibrosis stage, interleukin-28B (IL-28B) host genotype, age, and race. The model allowed differential risks for reinfection during incarceration and after release and for treatment initiation after release. We evaluated treatment strategies for 2 groups: persons with remaining sentences long enough to be eligible for 2- and 3-drug therapies (≥1.5 years; mean, 10 years) and those with remaining sentences too short to be eligible for treatment during incarceration (<1.5 years). We adopted a societal perspective in which we considered lifetime health benefits and costs regardless of to whom they accrued and discounted both at 3% annually (31, 32). Tables 1 and 2 of the Supplement (available at www.annals.org) show model inputs.
Starting Cohort
The model began with treatment-naive, 40-year-old men who had chronic, genotype 1 HCV monoinfection and were eligible for treatment, which is representative of most incarcerated persons (mean age, 40 years; 93% male [33]). We analyzed men because they account for the vast majority of incarcerated persons and sufficient published information is available on their mortality risks during and after incarceration and on reinfection. Although 34% of inmates are white, 39% are black, and 21% are Hispanic, the analysis considered only black and white inmates because data on effectiveness for Hispanic patients are limited. We stratified cohorts by race-specific IL-28B genotype because this predicts treatment response (18). Nearly 80% of HCV infections are genotype 1 (34). The liver disease distribution of the starting cohort, characterized by METAVIR scores ranging from F0 (no fibrosis) to F4 (compensated cirrhosis) (24), was based on studies of HCV-infected inmates (34). We analyzed patient subgroups in sensitivity analyses (Supplement).
Natural History
We focused on aspects of our model (24) that were unique to incarcerated populations (Figures 1 and 2 of the Supplement). Every 3 months, patients could have such health events as fibrosis progression. Treatment resulting in cure could leave patients with residual fibrosis consistent with their stage at the time but without additional progression, although this assumption was explored in sensitivity analyses. Untreated and uncured persons progressing to compensated cirrhosis were at risk for decompensated cirrhosis and hepatocellular carcinoma and could then become eligible for liver transplant. We assumed that progression rates in the absence of treatment were the same during incarceration and after release. Cured persons faced incarceration-specific risks for HCV reinfection. At all times, patients faced appropriate mortality risks.
Reinfection
During and after incarceration, persons may be reinfected despite spontaneous clearance or cure. Reinfection rates differ by incarceration status (Supplement) (26, 35). We assumed that reinfected patients continued liver fibrosis progression from the stage they reached before clearance or cure.
Mortality
Incarcerated persons have higher mortality risks than similar persons in the general population (36, 37). Patients with chronic HCV infection have higher mortality risks from liver-related and other causes (38). Data reported for inmates from 2001 to 2009 and life tables from the Centers for Disease Control and Prevention informed mortality rates specific to age, sex, race, and chronic HCV infection status (Table 3 of the Supplement) (39, 40). Previously published rates informed liver-related mortality among patients with advanced liver disease (22, 24). Prior studies have shown that SVR decreases mortality risks from liver-related causes (39, 40). On the basis of several large, long-term observational studies, we assumed that SVR decreased non–liver-related mortality risks by 10% (30, 41). We varied mortality risks in sensitivity analyses.
Incarceration
Although reinfection rates may be lower after release, early release can disrupt treatment. We modeled planned release and stratified the cohort by inmates with less than 1.5 years (short sentence) and at least 1.5 years (long sentence) left in their sentence at baseline (42). We modeled early release in the latter group by using government data (33). We assumed no release during HCV treatment and the effect of early release was that inmates could receive treatment earlier outside prison if there was no treatment program while they were incarcerated. We assumed that patients successfully treated in prison and released early had a lower risk for post-release reinfection (26, 35, 43, 44). Sensitivity analyses explored treatment disruption and reinfection.
Treatment During Incarceration
Treatment strategies during incarceration followed FDA-approved protocols and included no treatment, 2-drug therapy (pegylated interferon and ribavirin), or 3-drug therapy with either boceprevir or sofosbuvir (Supplement). For persons with long remaining sentences (42), we compared all strategies. For those with short sentences, we compared no treatment with sofosbuvir 3-drug therapy.
There is substantial interest in other regimens for which trials have shown high efficacy (8, 10, 45, 46). All-oral, interferon-sparing, sofosbuvir-based treatment (8, 10) has garnered attention because it may be better tolerated. We performed a scenario analysis in this fast-moving clinical area instead of including these treatments in the primary analysis because pricing information is not available for many of the treatments and they are currently not FDA-approved.
Postrelease Treatment
Previously incarcerated persons often have limited access to treatment (15). We conservatively assumed that persons treated after release received the most effective regimen (sofosbuvir 3-drug therapy) regardless of the treatment strategy offered during incarceration because this minimized the ascription of benefits to treatment during incarceration. Sensitivity analyses explored alternative assumptions.
Treatment Outcomes
For persons not achieving SVR after treatment, we did not consider retreatment because of evidence of low treatment uptake among formerly incarcerated persons (47) and a lack of retreatment data for sofosbuvir after prior unsuccessful sofosbuvir therapy. Treatment is contraindicated for approximately 17% of treatment-naive persons (48). We assumed that the 20-year cumulative probability of initiating postrelease treatment was 20% and increased it to 80% in sensitivity analyses. In a scenario analysis, we allowed early release during treatment and assumed that patients were unable to continue after release but could start treatment outside prison at the same cumulative probability as noted earlier. We assumed that close monitoring resulted in full treatment adherence during incarceration and varied this in sensitivity analyses. Postrelease adherence was assumed to be 80% of adherence during incarceration.
We stratified virologic response by race and IL-28B genotype for 2-drug therapy and boceprevir 3-drug therapy (24). For sofosbuvir 3-drug therapy, the NEUTRINO trial provided efficacy data stratified by IL-28B genotype, which did not vary substantially by race (7). We represented side-effect profiles collectively as regimen-specific quality-of-life decrements capturing treatment duration and side-effect severity (49). Interferon and ribavirin cause nausea, headache, and fatigue (50). Boceprevir increases anemia and rash (5). Sofosbuvir-based treatment has a reduced side-effect profile, potentially due to its short duration (7).
Quality of Life
Quality of life (QoL) was expressed as quality-adjusted life-years (QALYs). Aging reduces QoL (51), as does advancing liver disease (52). Depending on regimen, patients have temporary reductions during treatment. Achieving SVR improves QoL compared with pretreatment levels. Postrelease QoL changes are confined to those resulting from changes in treatment status or in chronic HCV–related health status.
Costs
We included background medical costs and costs related to chronic HCV infection and liver disease. Background medical costs were from estimates in U.S. correctional facilities and were adjusted by using age-specific medical cost patterns from the general population (14, 53). We further adjusted costs conditional on liver disease severity by accounting for nonliver comorbid conditions (54). Sensitivity analyses examined the effects of differing costs of health care delivery across correctional systems (14, 33). Background medical costs by age outside prison were similar to those in the general population (53). Tables 4 and 5 of the Supplement provide further details. Treatment costs are regimen-specific given differential drug costs and duration. We assumed that sofosbuvir cost $7000 per week (21) and adjusted to the Average Manufacturer Price with a factor of 0.64, and we explored alternative assumptions in sensitivity analyses (55). Costs are in 2013 U.S. dollars and were adjusted for inflation by using the Consumer Price Index (56).
Cost-Effectiveness
We assessed the value of each strategy by using incremental cost-effectiveness ratios (57), defined as the increase in cost for each additional unit of health benefit compared with the next best alternative.
Sensitivity Analyses
We assess the effect of alternate plausible assumptions and employed a probabilistic sensitivity analysis (PSA) to examine the impact of parameter uncertainty. The PSA, involved assigning distributions whose means and 95% CIs were identical to those in the primary analysis (Table 6 of the Supplement), obtaining 5000 repeated samples from all distributions and running analyses to characterize distributions of costs and benefits for each strategy, and determining the frequency at which each strategy was cost-effective at a given willingness-to-pay threshold.
Role of the Funding Source
The funding sources had no role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication.
Results
Sofosbuvir 3-drug therapy for treatment-naive, incarcerated men with chronic, genotype 1 HCV monoinfection is highly effective compared with alternative therapies. It resulted in an SVR of 85% for persons with short and long remaining sentences (Table), an absolute improvement of 73% compared with no treatment for those with short remaining sentences, and an absolute improvement of 26% compared with boceprevir 3-drug therapy for those with long remaining sentences.
Table.
Costs, Effectiveness, and Cost-Effectiveness Results of the Primary Analysis
| Strategy, by Remaining Sentence |
Cost, $ | SVR, % | Decompensated Cirrhosis, % |
Hepatocellular Carcinoma, % |
Liver Transplant, % |
QALYs | ICER, $/QALY |
|---|---|---|---|---|---|---|---|
| Short (<1.5 y) | |||||||
| No treatment in prison | 174,174 | 12 | 23 | 13 | 4 | 13.21 | - |
| Sofosbuvir 3-drug therapy | 228,316 | 85 | 6 | 4 | 1 | 15.31 | 25,700 |
| Long (≥1.5 y)* | |||||||
| No treatment in prison | 182,596 | 8 | 23 | 13 | 4 | 13.12 | - |
| 2-drug therapy | 227,832 | 28 | 19 | 11 | 3 | 13.57 | Extended dominance† |
| Boceprevir 3-drug therapy | 235,151 | 59 | 13 | 7 | 2 | 14.43 | Extended dominance† |
| Sofosbuvir 3-drug therapy | 241,948 | 85 | 7 | 4 | 1 | 15.18 | 28,800 |
ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year; SVR = sustained virologic response.
Small differences in rates of advanced liver disease (e.g., decompensated cirrhosis) among strategies for subgroups of incarcerated persons defined by length of remaining sentence are due to differential exposures to higher or lower rates of reinfection during incarceration and after release.
Costs more and provides fewer benefits than a combination of 2 strategies (in this case, no treatment in prison and sofosbuvir 3-drug therapy).
Sofosbuvir’s higher SVR rates produced such clinical benefits as reductions in decompensated cirrhosis (Table) a life-expectancy gain of 1.6 years compared with no treatment in prison for men with short remaining sentences, and a life-expectancy gain of 0.5 year compared with boceprevir 3-drug therapy for men with long remaining sentences (Table 7 of the Supplement).
Sofosbuvir increased discounted QALYs and costs compared with feasible alternatives. Although no treatment during incarceration yielded 13.21 and 13.12 QALYs for men with short and long remaining sentences, respectively, QALYs for 2-drug therapy and boceprevir 3-drug therapy were 13.57 and 14.43, respectively, for those with long remaining sentences (Table). Sofosbuvir-based therapy resulted in approximately 2.1 QALYs gained for men with short and long remaining sentences compared with no treatment during incarceration (Table and Figure). Costs increased by approximately $54 000 for men with short remaining sentences and $58 000 for those with long remaining sentences compared with no treatment during incarceration. For men with long remaining sentences, sofosbuvir 3-drug therapy dominated 2-drug therapy and boceprevir 3-drug therapy, achieving additional health benefits at a more favorable cost per QALY gained. Sofosbuvir 3-drug therapy cost $25 700 and $28 800 per QALY gained for men with short and long remaining sentences, respectively, compared with no treatment during incarceration. Costs per QALY gained differed between the groups because of differences in background medical costs and reinfection rates during and after incarceration.
Figure.
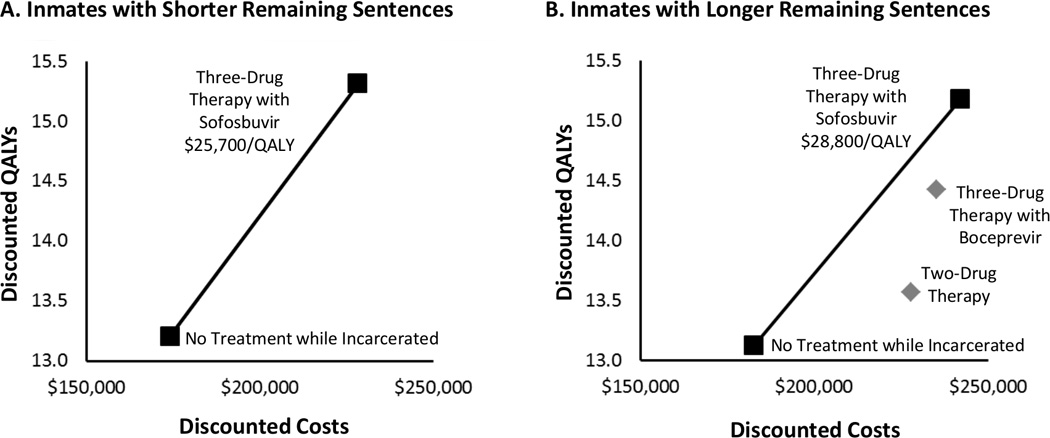
Cost-effectiveness results. The graphs show discounted QALYs (y-axis) and discounted total expected lifetime costs (x-axis) for each treatment strategy for inmates with short remaining sentences (<1.5 y) and those with long remaining sentences (≥1.5 y). The line segments make up the efficient frontier. The squares depict strategies on the frontier, and incremental cost-effectiveness ratios (i.e., the ratio of the additional costs of an intervention and its additional effects compared with the next best alternative) are reported. The diamonds represent strategies not on the efficient frontier, which cost more and provide less benefit than a strategy or combination of strategies on the frontier. QALY = quality-adjusted life-year.
Affordability and divided benefits represent challenges to delivering sofosbuvir 3-drug therapy to incarcerated populations. The additional costs of delivering sofosbuvir-based therapy to 500 000 incarcerated persons could exceed $27 billion to $30 billion (Table 8 of the Supplement). Although the upfront costs of treatment of incarcerated persons (approximately $32 billion) would fall to correctional systems, offsets (approximately $2 billion to $5 billion) would probably benefit other systems, such as Medicaid, especially for inmates with short sentences, although this would be less for persons more likely to be incarcerated again.
Sensitivity Analyses
Substantial uncertainty surrounds sofosbuvir because it has only recently become clinically available. Assuming that its effectiveness was 70% of that observed in trials, we found costs of $45 100 per QALY gained for men with short remaining sentences but $178 400 for those with long remaining sentences given the availability of boceprevir-based treatment (Figure 3 of the Supplement). Figure 4 of the Supplement shows the influence of sofosbuvir’s price on its cost-effectiveness. In a pessimistic scenario involving lower efficacy and a higher price as well as better access and care outside prison, sofosbuvir cost $89 100 per QALY gained for men with short remaining sentences compared with no treatment and $127 300 for those with long remaining sentences compared with boceprevir 3-drug treatment (Table 9 of the Supplement).
The cost-effectiveness of sofosbuvir was influenced by risks for reinfection that differ due to reincarceration (Table 10 of the Supplement) and by variation in prevalence and risk behaviors across prisons and communities (Table 11 of the Supplement). When reinfection rates during and after incarceration were 10 to 20 times higher than in the primary analysis—similar to rates in some prisons worldwide and among high-risk injection drug users (15, 26, 35)—sofosbuvir 3-drug therapy cost $80 100 per QALY gained for men with short remaining sentences and $170 300 per QALY gained for those with long remaining sentences compared with no treatment during incarceration.
In sensitivity analyses, sofosbuvir 3-drug treatment frequently cost less than $50 000 per QALY gained compared with no treatment during incarceration (Tables 12 and 13 of the Supplement).
There is excitement among clinicians about such new treatments as interferon-sparing, all-oral sofosbuvir regimens, although none are currently FDA-approved and pricing data are limited (8, 10). In a scenario analysis, we assumed 90% efficacy, 180% of treatment costs (price of sofosbuvir and simeprevir), a duration of 12 weeks, and no treatment-related disutility. All-oral therapy cost $831 000 per QALY gained compared with sofosbuvir 3-drug therapy. For persons who were interferon-intolerant, this strategy cost less than $39 000 per QALY gained for those with short remaining sentences and $49 000 per QALY gained for those with long remaining sentences. Drug prices, uptake, and other emerging therapies are important in considering new regimens (Table 14 of the Supplement).
Probabilistic Sensitivity Analysis
Sofosbuvir 3-drug therapy was optimal 99% of the time at a willingness-to-pay threshold of $50 000 per QALY gained and 100% of the time at a threshold of $63 000 (Figure 5 of the Supplement).
Discussion
In our analysis, sofosbuvir 3-drug therapy was highly effective in incarcerated, treatment-naive men with chronic, genotype 1 HCV monoinfection, including those whose remaining sentences were too short for other treatments. Sofosbuvir increased total expected cost per person by more than $54 000, but its additional benefits yielded a cost per QALY gained of less than $30 000.
The value of sofosbuvir derives from its high efficacy and short duration. Unexpected discharge from prison reduces treatment completion. Sofosbuvir remained the preferred strategy even at a 46.2% annual early release rate (10 times that in the primary analysis; 11% of inmates in 12 weeks). Correctional facilities may stipulate forgoing early release during HCV treatment.
Although our study focused on sofosbuvir, it comments on the arrival of several highly effective, short-duration HCV treatments, including those given orally and without interferon. Our exploratory analyses found that the value of all-oral, interferon-sparing regimens (8, 10) depends heavily on their pricing, their attractiveness for uptake and adherence, and the rising bar of other effective and less costly comparator regimens.
Expensive drugs, such as sofosbuvir, stress affordability and are victims of divided budget planning. Total additional costs from treating 500 000 incarcerated persons with HCV infection could reach $30 billion for the Federal Bureau of Prisons and other entities, with approximately $2 billion to $5 billion in savings accruing primarily after release to such entities as Medicaid. Such postrelease savings from better treatment during incarceration are unlikely to be fully captured by correctional systems, which may curtail adoption of an otherwise cost-effective intervention.
In settings with high reinfection rates during or after incarceration, the cost-effectiveness of sofosbuvir was attenuated. Although HCV prevalence (9.6% to 41.1%) and risk behaviors vary across systems (58), reinfection rates must be higher than 0.18 per person-year for the cost per QALY gained to exceed $100 000.
Our study builds on prior cost-effectiveness analyses of HCV treatments (24, 59–63). Two studies examined sofosbuvir. Petta and colleagues did so within the Italian health care system (59), and Younossi and associates focused on interferon-sparing regimens (60). Our study contributes in 2 ways: first, by focusing on incarcerated persons who may otherwise receive 2-drug therapy or no treatment, and second, by considering release from prison and its effects on treatment disruption and access to care and the effect of reinfection on cost-effectiveness in high-risk populations.
Although health outcome estimates from model-based HCV studies vary widely (for example, remaining QALYs range from 4.5 to >20) (24, 61, 64–66), our estimates were consistent after we accounted for important features of the population considered and the modeling methods used. Models that start with older patient cohorts and more advanced fibrosis, use higher discount rates, and have shorter time horizons generally estimate fewer remaining QALYs. Our model began with a middle-aged male population with a moderate fibrosis distribution and followed it over a lifetime with a 3% annual discount rate. However, our population is at high risk for mortality and reinfection, which many prior models have not considered. Tables 12 and 13 of the Supplement show the effect of varying such assumptions quantitatively (such as lower mortality or an older cohort).
Our study has limitations. Several patient subpopulations were excluded. Our analysis included men because they make up 93% of the incarcerated population and most studies relevant to mortality and reinfection of incarcerated populations are reported for them. We did not include Hispanic persons because trials have not reported efficacy stratified by Hispanic origin. Inmates co-infected with HIV or hepatitis B virus were not included because natural history of co-infection is complex, and although data on sofosbuvir’s effectiveness in this population are emerging, they are currently insufficient for accurate modeling of this group. Because the proportion of HCV-infected inmates co-infected with HIV is 14% (67), model-based analyses that incorporate co-infection should be undertaken when data availability permits (68).
We did not directly consider telaprevir 3-drug therapy in addition to boceprevir 3-drug therapy for clarity of presentation. In our previous cost-effectiveness evaluations of regimens containing boceprevir or telaprevir (24), we found that the former provides a more favorable cost-effectiveness profile, although we acknowledge the difficulty of direct comparisons of efficacy. Given that the regimens have similar efficacy, costs, and QoL reductions from side effects and given that sofosbuvir-containing regimens dominated boceprevir-containing regimens even in sensitivity analyses, we believe this decision is reasonable.
Our analysis was conducted from the societal perspective, although the costs we used are from heterogeneous sources and may not perfectly capture the opportunity costs of all resources. For example, we multiplied drug prices by 0.64 to reflect the negotiating power of larger prison systems.
Treatment of HCV in relation to release from prison and reincarceration is complex. We assumed that inmates who were released early during treatment did not continue. Better HCV care coordination across agencies could alter this, although shorter, highly effective regimens may make this less important. Our study did not explicitly model reincarceration (33) because data to properly do so for HCV-infected and cured persons are limited or unavailable. In sensitivity analyses examining immediate lifetime reincarceration, our primary findings were unchanged, which is reassuring.
In conclusion, for U.S. incarcerated men, sofosbuvir 3-drug therapy seems effective and provides good value compared with other interventions commonly deemed cost-effective. Its short duration enables treatment of inmates with short remaining sentences and decreases risks for disruption or discontinuation. Given the high price of sofosbuvir and the large population of incarcerated persons with chronic HCV infection, affordability may be an issue, although the cost-effectiveness of sofosbuvir merits consideration.
Supplementary Material
Acknowledgment
The authors thank Lauren Cipriano, Douglas K. Owens, Paul Barnett, Steve Asch, and other colleagues focused on HCV treatment within the U.S. Department of Veterans Affairs for their helpful conversations.
Financial Support: Dr. Holodniy was supported by intramural funding from the U.S. Department of Veterans Affairs. Dr. Goldhaber-Fiebert was supported in part by a Career Development Award (K01AG037593-01A1) from the National Institute on Aging of the National Institutes of Health.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Department of Veterans Affairs or the U.S. government.
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M14-0602.
Supplement. Additional Information on Data and Methods
References
- 1.Spaulding AC, Thomas DL. Screening for HCV infection in jails. JAMA. 2012;307:1259–1260. doi: 10.1001/jama.2012.374. [PMID: 22453565] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larney S, Kopinski H, Beckwith CG, Zaller ND, Jarlais DD, Hagan H, et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology. 2013;58:1215–1224. doi: 10.1002/hep.26387. [PMID: 23504650] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [PMID: 21745274] [DOI] [PubMed] [Google Scholar]
- 4.Ghany MG, Strader DB, Thomas DL, Seeff LB American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [PMID: 19330875] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poordad F, McCone J, Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [PMID: 21449783] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [PMID: 21696307] [DOI] [PubMed] [Google Scholar]
- 7.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [PMID: 23607594] [DOI] [PubMed] [Google Scholar]
- 8.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. [PMID: 24209977] [DOI] [PubMed] [Google Scholar]
- 9.Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, et al. Phase 2b trial of interferon-free therapy for hepatitis C virus genotype 1. N Engl J Med. 2014;370:222–232. doi: 10.1056/NEJMoa1306227. [PMID: 24428468] [DOI] [PubMed] [Google Scholar]
- 10.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–221. doi: 10.1056/NEJMoa1306218. [PMID: 24428467] [DOI] [PubMed] [Google Scholar]
- 11.Weinbaum C, Lyerla R, Margolis HS Centers for Disease Control and Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003;52:1–36. [PMID: 12562146] [PubMed] [Google Scholar]
- 12.Beck AJ, Maruschak LM. Hepatitis Testing and Treatment in State Prisons. Washington, DC: U.S. Department of Justice; 2004. [Google Scholar]
- 13.U.S. Department of Justice. The Federal Bureau of Prison’s Efforts to Manage Inmate Health Care. Washington, DC: U.S. Department of Justice; 2008. Accessed at www.justice.gov/oig/reports/BOP/a0808/final.pdf on 4 August 2014. [Google Scholar]
- 14.The Pew Charitable Trusts. Managing Prison Health Care Spending. Philadelphia: The Pew Charitable Trusts; 2014. Accessed at www.pewtrusts.org/en/research-and-analysis/reports/2014/05/15/managing-prison-health-care-spending on 4 August 2014. [Google Scholar]
- 15.Spaulding AS, Kim AY, Harzke AJ, Sullivan JC, Linas BP, Brewer A, et al. Impact of new therapeutics for hepatitis C virus infection in incarcerated populations. Top Antivir Med. 2013;21:27–35. [PMID: 23596276] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Schwarzinger M, Carrat F, Goldhaber-Fiebert JD. Cost effectiveness of fibrosis assessment prior to treatment for chronic hepatitis C patients. PLoS One. 2011;6(12):e26783. doi: 10.1371/journal.pone.0026783. [PMID: 22164204] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645–652. doi: 10.1053/jhep.2003.50364. [PMID: 12939591] [DOI] [PubMed] [Google Scholar]
- 18.Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120–129. e18. doi: 10.1053/j.gastro.2010.04.013. [PMID: 20399780] [DOI] [PubMed] [Google Scholar]
- 19.Fried MW, Buti M, Dore GJ, Flisiak R, Ferenci P, Jacobson I, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58:1918–1929. doi: 10.1002/hep.26641. [PMID: 23907700] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. FDA approves Sovaldi for chronic hepatitis C. Silver Spring, MD: U.S. Food and Drug Administration; 2013. Accessed at www.fda.gov/newsevents/newsroom/pressannouncements/ucm377888.htm on 1 February 2014. [Google Scholar]
- 21.Gilead. U.S. Food and Drug Administration Approves Gilead’s Sovaldi (Sofosbuvir) for the Treatment of Chronic Hepatitis C. Foster City, CA: Gilead; 2014. Accessed at www.gilead.com/news/press-releases/2013/12/us-food-and-drug-administration-approves-gileads-sovaldi-sofosbuvir-for-the-treatment-of-chronic-hepatitis-c on 21 June 2014. [Google Scholar]
- 22.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156:761–773. doi: 10.1093/aje/kwf100. [PMID: 12370165] [DOI] [PubMed] [Google Scholar]
- 23.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290:228–237. doi: 10.1001/jama.290.2.228. [PMID: 12851278] [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156:279–290. doi: 10.1059/0003-4819-156-4-201202210-00005. [PMID: 22351713] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutton AJ, Edmunds WJ, Gill ON. Estimating the cost-effectiveness of detecting cases of chronic hepatitis C infection on reception into prison. BMC Public Health. 2006;6:170. doi: 10.1186/1471-2458-6-170. [PMID: 16803622] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan JA, Joseph TA, Saab S. Treating hepatitis C in the prison population is cost-saving. Hepatology. 2008;48:1387–1395. doi: 10.1002/hep.22509. [PMID: 18924228] [DOI] [PubMed] [Google Scholar]
- 27.Arora S, Thornton K, Murata G, Deming P, Kalishman S, Dion D, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–2207. doi: 10.1056/NEJMoa1009370. [PMID: 21631316] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rice JP, Burnett D, Tsotsis H, Lindstrom MJ, Cornett DD, Voermans P, et al. Comparison of hepatitis C virus treatment between incarcerated and community patients. Hepatology. 2012;56:1252–1260. doi: 10.1002/hep.25770. [PMID: 22505121] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Schwarzinger M, Carrat F, Goldhaber-Fiebert JD. Cost effectiveness of fibrosis assessment prior to treatment for chronic hepatitis C patients. PLoS One. 2011;6:e26783. doi: 10.1371/journal.pone.0026783. [PMID: 22164204] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Cipriano LE, Holodniy M, Goldhaber-Fiebert JD. Cost-effectiveness analysis of risk-factor guided and birth-cohort screening for chronic hepatitis C infection in the United States. PLoS One. 2013;8:e58975. doi: 10.1371/journal.pone.0058975. [PMID: 23533595] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold M. Panel on cost-effectiveness in health and medicine. Med Care. 1996;34:DS197–DS199. [PMID: 8969326] [PubMed] [Google Scholar]
- 32.Weinstein MC, O'Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. ISPOR Task Force on Good Research Practices—Modeling Studies. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices—Modeling Studies. Value Health. 2003;6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [PMID: 12535234] [DOI] [PubMed] [Google Scholar]
- 33.Carson EA, Sabol WJ. Prisoners in 2011. Washington, DC: U.S. Department of Justice; 2012. pp. 1–23. Accessed at http://bjs.gov/content/pub/pdf/p11.pdf on 4 August 2014. [Google Scholar]
- 34.Sterling RK, Brown RS, Jr, Hofmann CM, Luketic VA, Stravitz RT, Sanyal AJ, et al. The spectrum of chronic hepatitis C virus infection in the Virginia Correctional System: development of a strategy for the evaluation and treatment of inmates with HCV. Am J Gastroenterol. 2005;100:313–321. doi: 10.1111/j.1572-0241.2005.40116.x. [PMID: 15667488] [DOI] [PubMed] [Google Scholar]
- 35.Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57(Suppl 2):S80–S89. doi: 10.1093/cid/cit306. [PMID: 23884071] [DOI] [PubMed] [Google Scholar]
- 36.Noonan ME, Carson EA. Prison and Jail Deaths in Custody, 2000–2009—Statistical Tables. Washington, DC: U.S. Department of Justice; 2011. [Google Scholar]
- 37.Arias E. United States Life Tables, 2009. Atlanta, GA: Centers for Disease Control and Prevention; 2014. National Vital Statistics Reports. [PubMed] [Google Scholar]
- 38.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clin Infect Dis. 2011;53:150–157. doi: 10.1093/cid/cir306. [PMID: 21665867] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516. e1. doi: 10.1016/j.cgh.2011.03.004. [PMID: 21397729] [DOI] [PubMed] [Google Scholar]
- 40.Innes HA, Hutchinson SJ, Allen S, Bhattacharyya D, Bramley P, Delahooke TE, et al. Hepatitis C Clinical Database Monitoring Committee. Excess liver-related morbidity of chronic hepatitis C patients, who achieve a sustained viral response, and are discharged from care. Hepatology. 2011;54:1547–1558. doi: 10.1002/hep.24561. [PMID: 22045672] [DOI] [PubMed] [Google Scholar]
- 41.Di Martino V, Crouzet J, Hillon P, Thévenot T, Minello A, Monnet E. Long-term outcome of chronic hepatitis C in a population-based cohort and impact of antiviral therapy: a propensity-adjusted analysis. J Viral Hepat. 2011;18:493–505. doi: 10.1111/j.1365-2893.2011.01476.x. [PMID: 21692956] [DOI] [PubMed] [Google Scholar]
- 42.Federal Bureau of Prisons. Sentences Imposed. Washington, DC: Federal Bureau of Prisons; 2014. Accessed at www.bop.gov/about/statistics/statistics_inmate_sentences.jsp on 4 August 2014. [Google Scholar]
- 43.Rowell TL, Wu E, Hart CL, Haile R, El-Bassel N. Predictors of drug use in prison among incarcerated black men. Am J Drug Alcohol Abuse. 2012;38:593–597. doi: 10.3109/00952990.2012.694536. [PMID: 22746253] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clarke JG, Stein MD, Hanna L, Sobota M, Rich JD. Active and former injection drug users report of HIV risk behaviors during periods of incarceration. Subst Abus. 2001;22:209–216. doi: 10.1080/08897070109511463. [PMID: 12466681] [DOI] [PubMed] [Google Scholar]
- 45.Poordad F, Lawitz E, Kowdley KV, Cohen DE, Podsadecki T, Siggelkow S, et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N Engl J Med. 2013;368:45–53. doi: 10.1056/NEJMoa1208809. [PMID: 23281975] [DOI] [PubMed] [Google Scholar]
- 46.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [PMID: 24795200] [DOI] [PubMed] [Google Scholar]
- 47.Baillargeon JG, Giordano TP, Harzke AJ, Baillargeon G, Rich JD, Paar DP. Enrollment in outpatient care among newly released prison inmates with HIV infection. Public Health Rep. 2010;125(Suppl 1):64–71. doi: 10.1177/00333549101250S109. [PMID: 20408389] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narasimhan G, Sargios TN, Kalakuntla R, Homel P, Clain DJ, Theise ND, et al. Treatment rates in patients with chronic hepatitis C after liver biopsy. J Viral Hepat. 2006;13:783–786. doi: 10.1111/j.1365-2893.2006.00763.x. [PMID: 17052279] [DOI] [PubMed] [Google Scholar]
- 49.Younossi ZM, Stepanova M, Henry L, Gane E, Jacobson IM, Lawitz E, et al. Minimal impact of sofosbuvir and ribavirin on health related quality of life in Chronic Hepatitis C (CH-C) J Hepatol. 2014;60:741–747. doi: 10.1016/j.jhep.2013.12.006. [PMID: 24333184] [DOI] [PubMed] [Google Scholar]
- 50.Fattovich G, Giustina G, Favarato S, Ruol A. A survey of adverse events in 11,241 patients with chronic viral hepatitis treated with alfa interferon. J Hepatol. 1996;24:38–47. doi: 10.1016/s0168-8278(96)80184-x. [PMID: 8834023] [DOI] [PubMed] [Google Scholar]
- 51.Nyman JA, Barleen NA, Dowd BE, Russell DW, Coons SJ, Sullivan PW. Quality-of-life weights for the US population: self-reported health status and priority health conditions, by demographic characteristics. Med Care. 2007;45:618–628. doi: 10.1097/MLR.0b013e31803dce05. [PMID: 17571010] [DOI] [PubMed] [Google Scholar]
- 52.McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making. 2008;28:582–592. doi: 10.1177/0272989X08315240. [PMID: 18424560] [DOI] [PubMed] [Google Scholar]
- 53.Meara E, White C, Cutler DM. Trends in medical spending by age, 1963–2000. Health Aff (Millwood) 2004;23:176–183. doi: 10.1377/hlthaff.23.4.176. [PMID: 15318578] [DOI] [PubMed] [Google Scholar]
- 54.Poret AW, Ozminkowski RJ, Goetzel R, Pew JE, Balent J. Cost burden of illness for hepatitis C patients with employer-sponsored health insurance. Dis Manag. 2002;5:95–107. [Google Scholar]
- 55.Holtz-Eakin D. Prices for Brand-Name Drugs Under Selected Federal Programs. Washington, DC: Congressional Budget Office; 2005. Accessed at www.cbo.gov/ftpdocs/64xx/doc6481/06-16-PrescriptDrug.pdf on 1 September 2011. [Google Scholar]
- 56.U.S. Department Of Labor. Bureau of Labor Statistics Consumer Price Index. Accessed at http://www.bls.gov/cpi/ on 1 January 2014.
- 57.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford Univ Pr; 1996. [Google Scholar]
- 58.Varan A, Mercer D, Stein M, Spaulding A. State prison system surveillance of hepatitis C exposure: limited data show declining share of US epidemic [Poster abstract]. Presented at Fifth Academic and Health Policy Conference on Correctional Health; 22–23 March 2012; Atlanta, GA. [Google Scholar]
- 59.Petta S, Cabibbo G, Enea M, Macaluso FS, Plaia A, Bruno R, et al. WEF Study Group. Cost-effectiveness of sofosbuvir-based triple therapy for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2014;59:1692–1705. doi: 10.1002/hep.27010. [PMID: 24691835] [DOI] [PubMed] [Google Scholar]
- 60.Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60:530–537. doi: 10.1016/j.jhep.2013.11.009. [PMID: 24269472] [DOI] [PubMed] [Google Scholar]
- 61.Cammà C, Petta S, Enea M, Bruno R, Bronte F, Capursi V, et al. WEF Study Group. Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2012;56:850–860. doi: 10.1002/hep.25734. [PMID: 22454336] [DOI] [PubMed] [Google Scholar]
- 62.Blázquez-Pérez A, San Miguel R, Mar J. Cost-effectiveness analysis of triple therapy with protease inhibitors in treatment-naive hepatitis C patients. Pharmacoeconomics. 2013;31:919–931. doi: 10.1007/s40273-013-0080-3. [PMID: 24000086] [DOI] [PubMed] [Google Scholar]
- 63.Elbasha EH, Chhatwal J, Ferrante SA, El Khoury AC, Laires PA. Cost-effectiveness analysis of boceprevir for the treatment of chronic hepatitis C virus genotype 1 infection in Portugal. Appl Health Econ Health Policy. 2013;11:65–78. doi: 10.1007/s40258-012-0007-8. [PMID: 23355388] [DOI] [PubMed] [Google Scholar]
- 64.Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J. Pegylated interferon alpha-2a and-2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2004;8:iii–iv. 1–125. doi: 10.3310/hta8390. [PMID: 15461877] [DOI] [PubMed] [Google Scholar]
- 65.Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–205. iii. doi: 10.3310/hta11110. [PMID: 17346498] [DOI] [PubMed] [Google Scholar]
- 66.Hartwell D, Jones J, Baxter L, Shepherd J. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess. 2011;15:i–xii. 1–210. doi: 10.3310/hta15170. [PMID: 21473834] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weinbaum CM, Sabin KM, Santibanez SS. Hepatitis B, hepatitis C, HIV in correctional populations: a review of epidemiology and prevention. AIDS. 2005;19(Suppl 3):S41–S46. doi: 10.1097/01.aids.0000192069.95819.aa. [PMID: 16251827] [DOI] [PubMed] [Google Scholar]
- 68.Cipriano LE, Zaric GS, Holodniy M, Bendavid E, Owens DK, Brandeau ML. Cost effectiveness of screening strategies for early identification of HIV and HCV infection in injection drug users. PLoS One. 2012;7:e45176. doi: 10.1371/journal.pone.0045176. [PMID: 23028828] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


